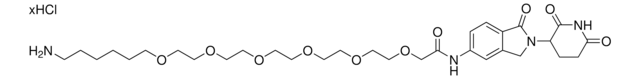
921300
C5 Lenalidomide-pyrimidine-piperazine-PEG1-NH2 hydrochloride
≥95%
同義詞:
5-(4-(2-(2-Aminoethoxy)ethyl)piperazin-1-yl)-N-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-5-yl)pyrimidine-2-carboxamide hydrochloride, Crosslinker−E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
推薦產品
ligand
C5 Lenalidomide
品質等級
化驗
≥95%
形狀
(Powder or crystals or flakes or chunks)
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
儲存溫度
2-8°C
SMILES 字串
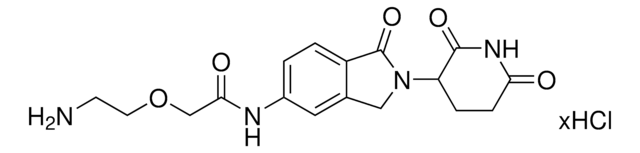
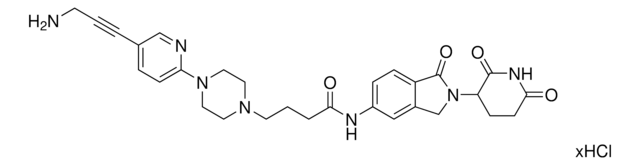
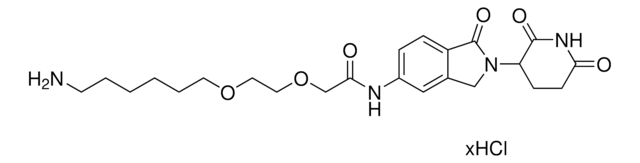
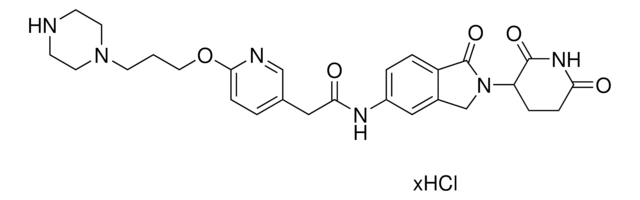
O=C1N(C2CCC(NC2=O)=O)CC3=CC(NC(C4=NC=C(N5CCN(CCOCCN)CC5)C=N4)=O)=CC=C31.Cl
應用
其他說明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
法律資訊
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
文章
Protein Degrader Building Blocks 是一系列交聯劑-E3 配體結合物,具有懸垂功能基團,可與目標配體共價連結。
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務