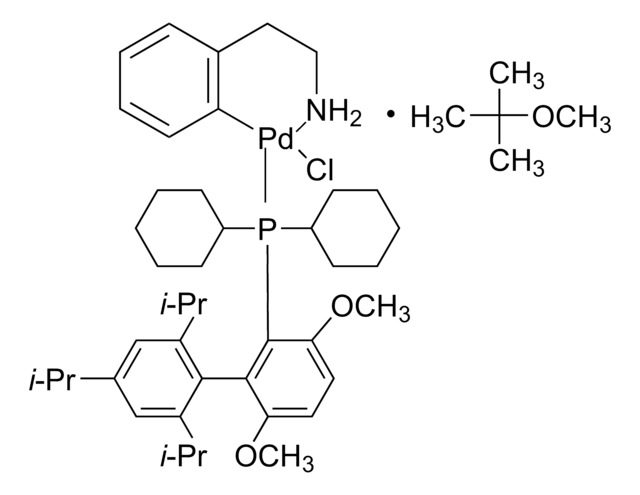
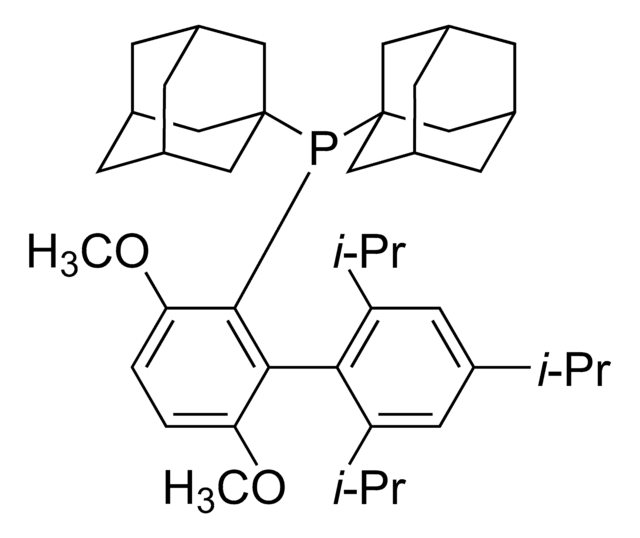
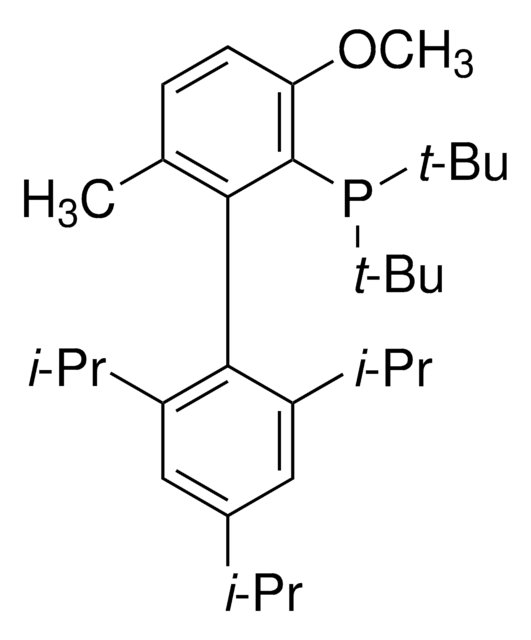
推薦產品
品質等級
化驗
96%
形狀
solid
特點
generation 3
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
119-131 °C
官能基
phosphine
SMILES 字串
COC1=CC=C(C(P(C(C)(C)C)C(C)(C)C)=C1C2=C(C=C(C=C2C(C)C)C(C)C)C(C)C)OC.NC3=C(C=CC=C3)C4=C(C=CC=C4)[Pd]OS(C)(=O)=O
InChI
1S/C31H49O2P.C12H10N.CH4O3S.Pd/c1-19(2)22-17-23(20(3)4)27(24(18-22)21(5)6)28-25(32-13)15-16-26(33-14)29(28)34(30(7,8)9)31(10,11)12;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h15-21H,1-14H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChI 密鑰
GAQPAUHHECNHNS-UHFFFAOYSA-M
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
文章
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
G3和G4 Buchwald预催化剂是一类最新的空气、湿度和热稳定型交叉偶联复合物,可用于键形成以实现其多功能性和高反应性。
相關內容
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務





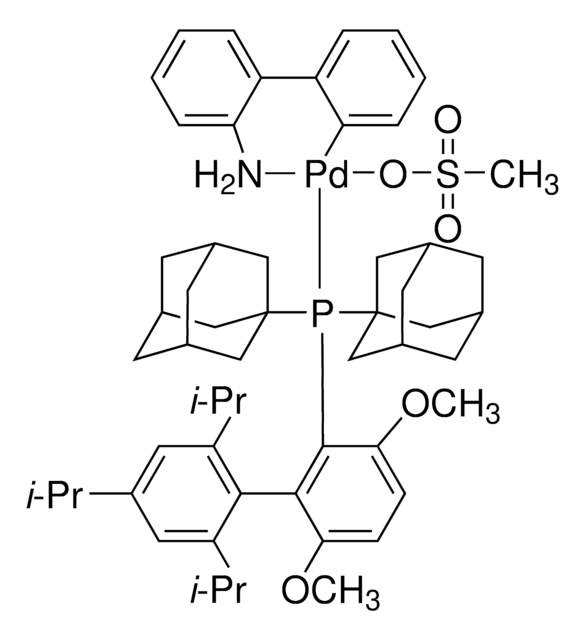

![[(二(1-金刚烷基)丁基膦基)-2-(2′-氨基-1,1′-联苯基)]钯(II)甲磺酸酯 95%](/deepweb/assets/sigmaaldrich/product/structures/391/492/af15708b-9501-44ae-a25f-d3574589a865/640/af15708b-9501-44ae-a25f-d3574589a865.png)