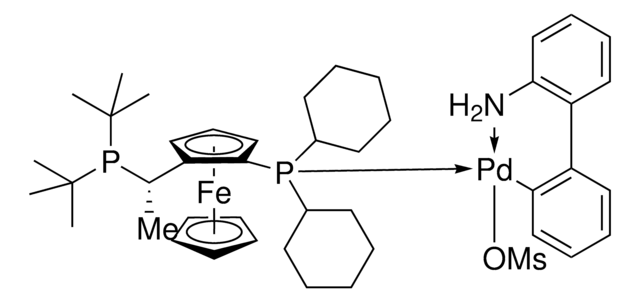
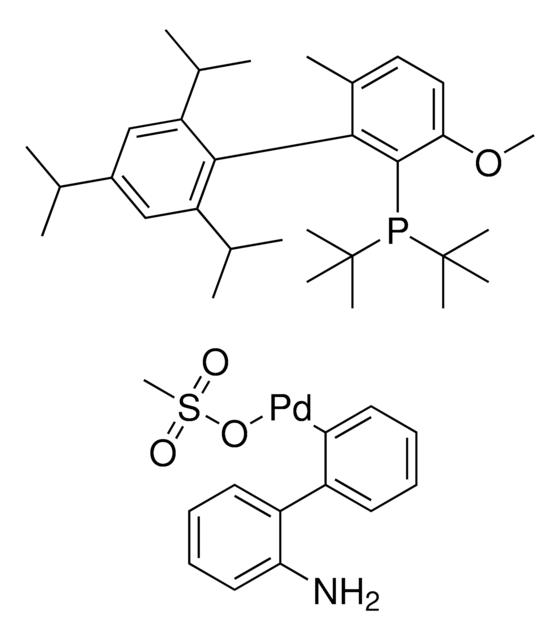
推薦產品
品質等級
化驗
98%
形狀
solid
特點
generation 3
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: catalyst
core: palladium
reaction type: Cross Couplings
mp
150-193 °C (decomposition)
官能基
amine
SMILES 字串
CS(=O)(O[Pd]c1c(c2c(N)cccc2)cccc1)=O.COc3c(P(C4CCCCC4)C5CCCCC5)c(c6c(C(C)C)cc(C(C)C)cc6C(C)C)c(OC)cc3
InChI
1S/C35H53O2P.C12H10N.CH4O3S.Pd/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h19-25,27-28H,9-18H2,1-8H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChI 密鑰
PQYBJDCHLVJYSD-UHFFFAOYSA-M
一般說明
應用
可用于合成下列化合物:
- 与[(肉桂基)PdCl]2和AgOTf反应生成[Pd(肉桂基)(BrettPhos)]OTf。
- 与[(巴豆基)PdCl]2和AgOTf反应生成[Pd(巴豆基)(BrettPhos)]OTf。
- 与[(烯丙基)PdCl]2和AgOTf反应生成[Pd(烯丙基)(BrettPhos)]OTf。
- 与[(烯丙基)PdCl]2反应生成Pd(烯丙基)(BrettPhos)Cl。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
分析證明 (COA)
客戶也查看了
文章
Preformed catalysts in the kit are stable but become air-sensitive when activated; Schlenk technique aids scale-up.
套件中的預成型催化劑是穩定的,但在活化時會變得對空氣敏感;Schlenk 技術有助於擴展規模。
Preformed catalysts in the kit are stable but become air-sensitive when activated; Schlenk technique aids scale-up.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
相關內容
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務






![[(二(1-金刚烷基)丁基膦基)-2-(2′-氨基-1,1′-联苯基)]钯(II)甲磺酸酯 95%](/deepweb/assets/sigmaaldrich/product/structures/391/492/af15708b-9501-44ae-a25f-d3574589a865/640/af15708b-9501-44ae-a25f-d3574589a865.png)