推薦產品
品質等級
化驗
97%
形狀
solid
特點
generation 1
反應適用性
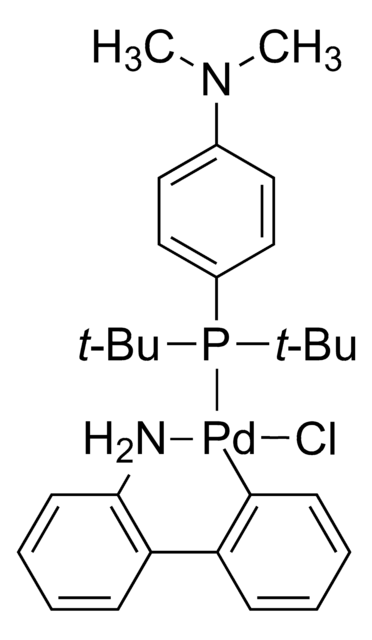
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
198-204 °C
官能基
phosphine
SMILES 字串
COC(C)(C)C.NCCc1ccccc1[Pd]Cl.COc2ccc(OC)c(c2P(C3CCCCC3)C4CCCCC4)-c5c(cc(cc5C(C)C)C(C)C)C(C)C
InChI
1S/C35H53O2P.C8H10N.C5H12O.ClH.Pd/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28;9-7-6-8-4-2-1-3-5-8;1-5(2,3)6-4;;/h19-25,27-28H,9-18H2,1-8H3;1-4H,6-7,9H2;1-4H3;1H;/q;;;;+1/p-1
InChI 密鑰
OWHWOTGYDWMPCA-UHFFFAOYSA-M
一般說明
應用
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
相關內容
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務





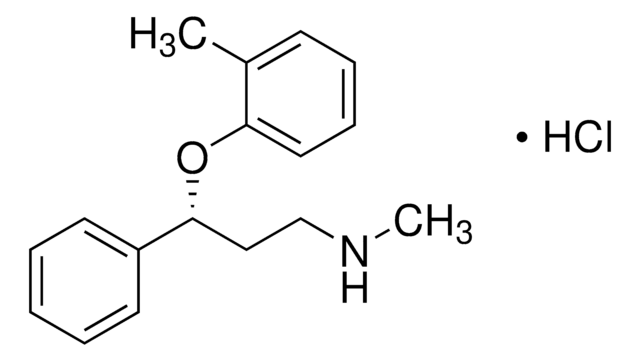


 95%](/deepweb/assets/sigmaaldrich/product/structures/151/609/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1/640/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1.png)