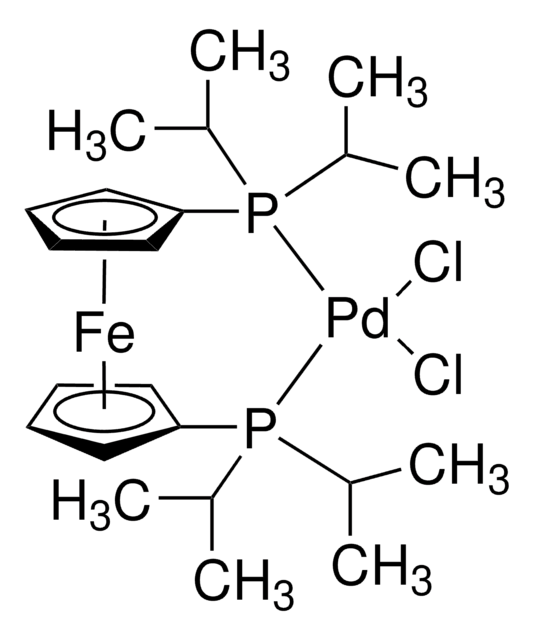
About This Item
推薦產品
形狀
solid
品質等級
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
官能基
phosphine
SMILES 字串
Cl[Pd]Cl.CN(c1ccc(P(C(C)(C)C)C(C)(C)C)cc1)C.CN(c2ccc(P(C(C)(C)C)C(C)(C)C)cc2)C
InChI
1S/2C16H28NP.2ClH.Pd/c2*1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;;;/h2*9-12H,1-8H3;2*1H;/q;;;;+2/p-2
InChI 密鑰
DWOZNANUEDYIOF-UHFFFAOYSA-L
相關類別
一般說明
对于小规模和高通量用途,产品为ChemBeads(927791)
應用
- 通过氨钯诱导的Heck型反应,手性选择性构建吲哚-融合双环[3.2.1]-辛烷。
- 通过Suzuki交叉偶联反应后的分子内缩合反应,从邻溴N-甲苯磺酰腙和2-氨基苯硼酸酯合成苯并咪唑衍生物
- 通过邻卤苯醇和内炔环化pd催化合成茚酮。
訊號詞
Warning
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
從最近期的版本中選擇一個:
分析證明 (COA)
客戶也查看了
文章
Ligand used to prepare a palladium dichloride catalyst on treatment with PdCl2(COD). The catalyst effectively cross-couples aryl boronic acids with heteroaryl chlorides.
A variety of palladium-catalyzed cross-coupling reactions can be run under room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
條款
TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
TPGS-750-M 表面活化劑可在室溫下於水中進行各種反應,提高合成的效率與多樣性。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![[1,1′-双(二-叔丁基膦基)二茂铁]二氯合钯(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[(二(1-金刚烷基)丁基膦基)-2-(2′-氨基-1,1′-联苯基)]钯(II)甲磺酸酯 95%](/deepweb/assets/sigmaaldrich/product/structures/391/492/af15708b-9501-44ae-a25f-d3574589a865/640/af15708b-9501-44ae-a25f-d3574589a865.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)