推薦產品
蒸汽密度
>1 (vs air)
蒸汽壓力
<0.01 mmHg ( 20 °C)
化驗
98%
形狀
liquid
折射率
n20/D 1.578 (lit.)
bp
278-282 °C/760 mmHg (lit.)
密度
1.094 g/mL at 25 °C (lit.)
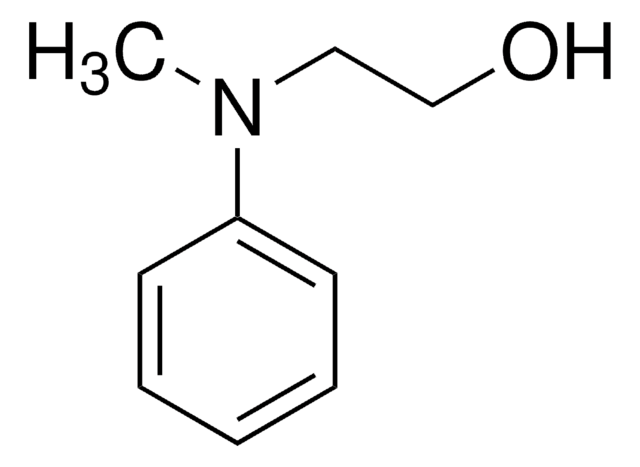
SMILES 字串
OCCNc1ccccc1
InChI
1S/C8H11NO/c10-7-6-9-8-4-2-1-3-5-8/h1-5,9-10H,6-7H2
InChI 密鑰
MWGATWIBSKHFMR-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
N-(2-羟乙基)苯胺被用作人嗅觉UDP-葡糖醛酸糖基转移酶的底物。[1]
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - STOT RE 2 - STOT SE 1
標靶器官
Blood, Blood,hematopoietic system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Santos Fustero et al.
The Journal of organic chemistry, 74(11), 4429-4432 (2009-05-15)
The preparation of cyclic dipeptide mimetics from chiral imino lactones derived from (R)-phenylglycinol is described. Key steps of the synthetic route included the fully stereoselective construction of a quaternary center, the formation of six-, seven-, or eight-membered lactams by means
N Philippe et al.
Organic letters, 2(15), 2185-2187 (2000-08-10)
Highly diastereoselective protonation of chiral lactam enolates of 4-substituted-1,4-dihydroisoquinolin-3-ones is reported. Protonation and alkylation processes of these lactam enolates derived from phenylglycinol occur with opposite diastereofacial selectivity. This diastereoselective protonation has been applied to the asymmetric synthesis of (4S)-N-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline 9
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(30), 7872-7881 (2006-07-20)
A straightforward procedure for the synthesis of enantiopure polysubstituted piperidines is reported. It involves the direct generation of chiral non-racemic oxazolo[3,2-a]piperidone lactams that already incorporate carbon substituents on the heterocyclic ring and the subsequent removal of the chiral auxiliary. The
Mercedes Amat et al.
The Journal of organic chemistry, 71(10), 3804-3815 (2006-05-06)
The stereochemical outcome of the alkylation of a variety of phenylglycinol-derived oxazolopiperidone lactams is studied. The influence of the configuration of the C-8a stereocenter and the effect of the substituents at the C-8 and C-8a positions on the stereoselectivity of
M Amat et al.
Organic letters, 3(21), 3257-3260 (2001-10-12)
[reaction: see text]. The phenylglycinol-derived 2-pyridone 1 undergoes m-CPBA oxidation steroselectively leading to the chiral nonracemic unsaturated bicyclic hydroxylactam 2, from which the enantioselective synthesis of (3R,5R)-3,4,5-trihydroxypiperidine (16) and the formal synthesis of the azasugar epiisofagomine are described. The enantioselective
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務