推荐产品
等級
anhydrous
品質等級
agency
suitable for EPA 1613
蒸汽密度
2.9 (vs air)
蒸汽壓力
24.45 psi ( 55 °C)
6.83 psi ( 20 °C)
6.86 psi ( 20 °C)
化驗
≥99.8%
形狀
liquid
自燃溫度
1223 °F
包含
40-150 ppm amylene as stabilizer
expl. lim.
22 %
技術
gas chromatography (GC): suitable
雜質
≤0.001% water
≤0.005% water (100 mL pkg)
蒸發殘留物
<0.0005%
折射率
n20/D 1.424 (lit.)
bp
39.8-40 °C (lit.)
mp
−95 °C (lit.)
密度
1.325 g/mL at 25 °C (lit.)
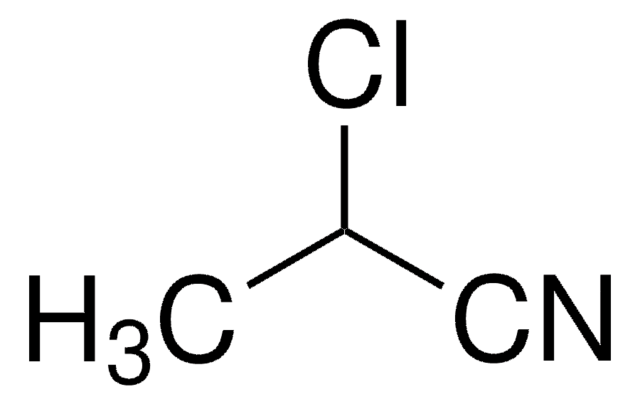
SMILES 字串
ClCCl
InChI
1S/CH2Cl2/c2-1-3/h1H2
InChI 密鑰
YMWUJEATGCHHMB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
包裝
也與該產品經常一起購買
相關產品
訊號詞
Warning
危險分類
Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
does not flash
閃點(°C)
does not flash
個人防護裝備
Eyeshields, Faceshields, Gloves
其他客户在看
商品
Substances are said to be miscible in one another if they dissolve to form a uniform solution. Bookmark or download our miscibility table for common lab solvents.
相关内容
This page is intended to make it easier to find the consumables you need based on the analytical method you’re using. Methods included on this page come from the EPA, Standard Methods and ASTM.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门