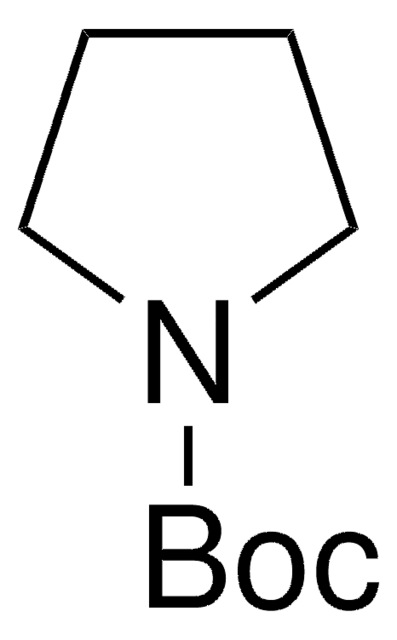
所有图片(1)
About This Item
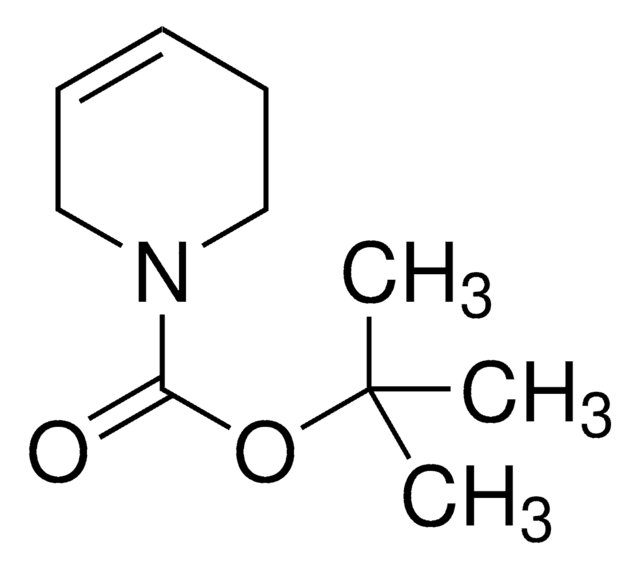
经验公式(希尔记法):
C9H15NO2
CAS号:
分子量:
169.22
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
折射率
n20/D 1.458 (lit.)
bp
208 °C (lit.)
密度
0.981 g/mL at 25 °C (lit.)
SMILES 字串
CC(C)(C)OC(=O)N1CC=CC1
InChI
1S/C9H15NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-5H,6-7H2,1-3H3
InChI 密鑰
YEBDZDMYLQHGGZ-UHFFFAOYSA-N
一般說明
N-Boc-2,5-dihydro-1H-pyrrole, also known as tert-butyl 2,5-dihydro-1H-pyrrole-1-carboxylate, can be synthesized from N-boc-diallylamine.
應用
用于与芳香重氮盐通过 Heck 芳基化反应合成 ß-芳基-GABA 类似物。
N-Boc-2,5-dihydro-1H-pyrrole (tert-Butyl 2,5-dihydro-1H-pyrrole-1-carboxylate) may be used in the preparation of tert-butyl 3-aryl-2,3-dihydro-1H-pyrrole-1-carboxylate.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
178.0 °F
閃點(°C)
81.1 °C
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
A Short and Efficient Synthesis of (S)-1-Boc-2, 5-dihydro-1H-pyrrole-2-carboxylic Acid.
Sturmer R, et al.
Synthesis, 1, 46-48 (2001)
Synthesis of aryl pyrrolizidines from endocyclic enecarbamates. Novel applications of the Heck arylation of 3-pyrrolines using diazonium salts.
de Oca ACBM and Correia CRD.
ARKIVOC (Gainesville, FL, United States), 10, 390-403 (2003)
Ariel L L Garcia et al.
The Journal of organic chemistry, 70(3), 1050-1053 (2005-01-29)
We report herein a new, practical, and economic synthesis of the phosphodiesterase inhibitor Rolipram on a multigram scale as well as the synthesis of new 4-aryl pyrrolidones and beta-aryl-gamma-amino butyric acids (GABA derivatives) employing an efficient Heck-Matsuda arylation of 3-pyrroline
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![(1S,4S)-(-)-2-Boc-2,5-二氮杂双环[2.2.1]庚烷 95%](/deepweb/assets/sigmaaldrich/product/structures/401/063/1eb3be94-6385-4815-8b42-ff76c097cc27/640/1eb3be94-6385-4815-8b42-ff76c097cc27.png)