E6754
Epoxy-activated−Sepharose™ 6B
lyophilized powder
Synonim(y):
Epoxy-activated-Agarose
About This Item
Polecane produkty
Postać
lyophilized powder
zakres etykietowania
19-40 μmol per mL
metody
affinity chromatography: suitable
macierz
Sepharose 6B
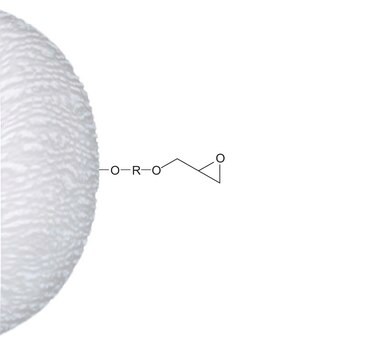
grupa aktywna macierzy
epoxy
MAR
through epoxy to hydroxyl
spacer macierzy
12 atoms
pęcznienie
1 g swells to 3.0-5.0 mL
wielkość cząstki
45—165 μm
temp. przechowywania
2-8°C
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Inne uwagi
Attachment: one epoxy group
Spacer: provides a 12-atom spacer when ligands are coupled to free epoxy groups. Linkage is a stable, uncharged ether.
Postać fizyczna
Informacje prawne
zastąpiony przez
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej