70050
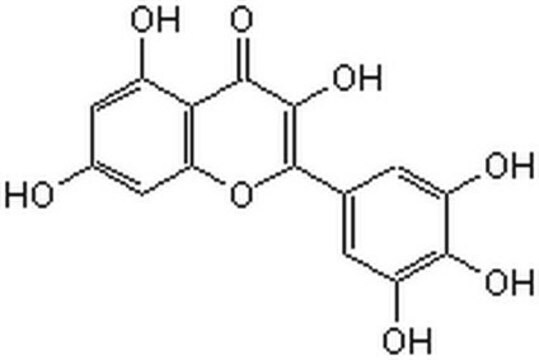
Myricetin
≥96.0% (HPLC)
Synonim(y):
3,3′,4′,5,5′,7-Hexahydroxyflavone, Cannabiscetin, Myricetol
About This Item
Polecane produkty
Próba
≥96.0% (HPLC)
Postać
powder
mp
≥300 °C
>300 °C (lit.)
rozpuszczalność
ethanol: 10 mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow
Zastosowanie
metabolomics
vitamins, nutraceuticals, and natural products
ciąg SMILES
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
Klucz InChI
IKMDFBPHZNJCSN-UHFFFAOYSA-N
informacje o genach
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
- to study its preventive effect as an antioxidant on noise-induced hearing loss (NIHL) in rats
- as a flavonoid compound to test antiviral activity of Bourbon virus (BRBV) and in inhibition of RNA-dependent RNA polymerase (RdRP)
- to study its effect as a treatment on biofilms of Streptococcus mutans and Candida albicans
- as a reference standard for the quantification of phenolic compounds from Juniperus species
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protokoły
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej