MAB8251
Anti-Influenza A Antibody, nucleoprotein, clones A1, A3 Blend
ascites fluid, Chemicon®, from mouse
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Kod UNSPSC:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Polecane produkty
pochodzenie biologiczne
mouse
Poziom jakości
forma przeciwciała
ascites fluid
rodzaj przeciwciała
primary antibodies
klon
A1, monoclonal
A3, monoclonal
reaktywność gatunkowa
human
producent / nazwa handlowa
Chemicon®
metody
immunofluorescence: suitable
izotyp
IgG1 (A3)
IgG2a (A1)
Warunki transportu
wet ice
Specyficzność
All subtypes of Influenza A (H1N1, H2N2 and H3N2 and H5N1). Specific for the nucleoprotein antigen. No cross-reactivity seen to Influenza B, Parainfluenza 1, 2 or Adenovirus or RSV.
Immunogen
Influenza A blend.
Zastosowanie
Indirect immunofluorescence at 1:100-1:1000.
Optimal working dilutions must be determined by end user.
Optimal working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
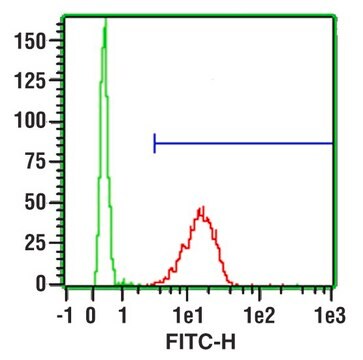
This Anti-Influenza A Antibody, nucleoprotein, clones A1, A3 Blend is validated for use in IF for the detection of Influenza A.
Postać fizyczna
Ascites fluid containing no preservatives.
Unpurified
Przechowywanie i stabilność
Maintain for 1 year at -20°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
Komentarz do analizy
Control
Influenza Control Slides, Catalogue Number 5010-5
Influenza Control Slides, Catalogue Number 5010-5
Inne uwagi
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Informacje prawne
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Oświadczenie o zrzeczeniu się odpowiedzialności
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
This page may contain text that has been machine translated.
Not finding the right product?
Try our Narzędzie selektora produktów.
polecane
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
nwg
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Validation of a method for preparing influenza H5N1 simulated samples.
John A Lednicky,Julie M Villanueva,Stephen A Burke,Roxanne Shively,Michael W Shaw et al.
Journal of Virological Methods null
Rui Jia et al.
Cellular microbiology, 16(7), 1080-1093 (2014-02-14)
Members of the interferon-induced transmembrane (IFITM) protein family inhibit the entry of a wide range of viruses. Viruses often exploit the endocytosis pathways to invade host cells and escape from the endocytic vesicles often in response to low pH. Localization
Nara Lee et al.
Nucleic acids research, 45(15), 8968-8977 (2017-09-16)
Influenza A virus (IAV) genomes are composed of eight single-stranded RNA segments that are coated by viral nucleoprotein (NP) molecules. Classically, the interaction between NP and viral RNA (vRNA) is depicted as a uniform pattern of 'beads on a string'.
Shuguang Han et al.
Clinical and experimental pharmacology & physiology, 45(1), 84-93 (2017-08-31)
Curcumin, an active phenolic agent extract from the Curcuma longa, exhibits excellent anti-cancer, anti-inflammation, and neuroprotective effects. We aimed to investigate the anti-influenza role of curcumin in vitro and in vivo. The effect of curcumin on replication of influenza A
Recipients of vaccine against the 1976 "swine flu" have enhanced neutralization responses to the 2009 novel H1N1 influenza virus.
McCullers, JA; Van De Velde, LA; Allison, KJ; Branum, KC; Webby, RJ; Flynn, PM
Clinical Infectious Diseases null
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej