AB1056
Anti-Adenovirus Antibody
Chemicon®, from goat
About This Item
Polecane produkty
pochodzenie biologiczne
goat
Poziom jakości
forma przeciwciała
purified antibody
rodzaj przeciwciała
primary antibodies
klon
polyclonal
reaktywność gatunkowa
human
producent / nazwa handlowa
Chemicon®
metody
ELISA: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
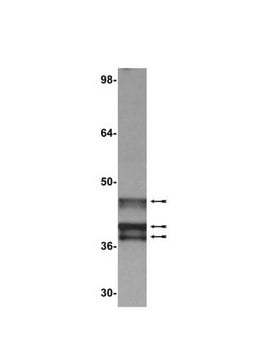
western blot: suitable
Warunki transportu
wet ice
Specyficzność
Immunogen
Zastosowanie
Fluorescence microscopy, indirect.
Immunoblotting: unboiled samples in 1mM DTT and 2% SDS only
Immunohistochemistry
Optimal working dilutions must be determined by end user
Infectious Diseases
Infectious Diseases - Viral
Postać fizyczna
Przechowywanie i stabilność
Inne uwagi
Informacje prawne
Oświadczenie o zrzeczeniu się odpowiedzialności
Not finding the right product?
Try our Narzędzie selektora produktów.
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej