475841
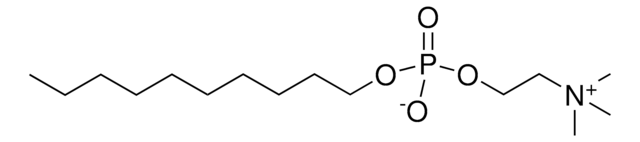
Miltefosine
An ether lipid analog that acts as an inhibitor of CTP:phosphocholine cytidyltransferase and displays remarkable antiproliferative effect both in vitro and in vivo.
Synonim(y):
Miltefosine, HePC, 1-Hexadecylphosphorylcholine
About This Item
Polecane produkty
Poziom jakości
Próba
≥98% (TLC)
Postać
crystalline solid
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
protect from light
rozpuszczalność
ethanol: 1 mg/mL
PBS, pH 7.2: 2.5 mg/mL
DMSO: 800 μg/mL
Warunki transportu
ambient
temp. przechowywania
−20°C
InChI
1S/C21H46NO4P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-27(23,24)26-21-19-22(2,3)4/h5-21H2,1-4H3
Klucz InChI
PQLXHQMOHUQAKB-UHFFFAOYSA-N
Opis ogólny
Działania biochem./fizjol.
CTP:phosphocholine cytidyltransferase
Opakowanie
Ostrzeżenie
Rekonstytucja
Inne uwagi
Wieder, T., et al. 1993. Biochem. J.291, 561.
Geilen, C.C., et al. 1992. J. Biol. Chem.267, 6719.
Geilen, C.C., et al. 1991. Eur. J. Cancer27, 1650.
Informacje prawne
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Oral
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej