H4405
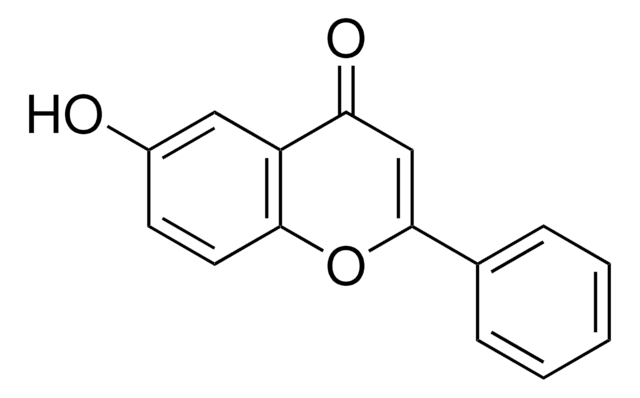
5-Hydroxyflavone
≥97%
Synonim(y):
5-Hydroxy-2-phenylchromone, NSC 26745, Primuletin
About This Item
Polecane produkty
Próba
≥97%
ciąg SMILES
Oc1cccc2OC(=CC(=O)c12)c3ccccc3
InChI
1S/C15H10O3/c16-11-7-4-8-13-15(11)12(17)9-14(18-13)10-5-2-1-3-6-10/h1-9,16H
Klucz InChI
IYBLVRRCNVHZQJ-UHFFFAOYSA-N
informacje o genach
rat ... Gabra2(29706)
Zastosowanie
- Condensation reactions for synthesis of copper(II) complexes as bioactive molecules to combat antioxidants
- Thermal behavior studies of vanadyl complexes with flavone derivatives in terms of insulin-mimetic agents
- O-methylation with di-Me carbonate
- DFT studies on excited-state intramolecular proton transfer
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej