428949
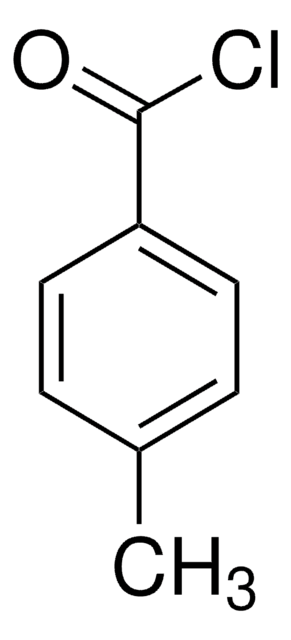
4-Methyl-3-nitrobenzoyl chloride
99%
Synonim(y):
3-Nitro-p-toluoyl chloride
About This Item
Polecane produkty
Próba
99%
Postać
liquid
współczynnik refrakcji
n20/D 1.581 (lit.)
tw
185 °C/36 mmHg (lit.)
mp
20-21 °C (lit.)
gęstość
1.37 g/mL at 25 °C (lit.)
ciąg SMILES
Cc1ccc(cc1[N+]([O-])=O)C(Cl)=O
InChI
1S/C8H6ClNO3/c1-5-2-3-6(8(9)11)4-7(5)10(12)13/h2-4H,1H3
Klucz InChI
DXMHBBURYDVYAI-UHFFFAOYSA-N
Opis ogólny
Zastosowanie
It may be used in the synthesis of the following:
- benzophenone derivative
- substituted 3-amino-4-methyl-N-phenylbenzamide
- retroamide
- 4-methyl-3-nitro-N-phenylbenzamide
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Dam. 1 - Skin Corr. 1B
Kod klasy składowania
8A - Combustible corrosive hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
235.4 °F - closed cup
Temperatura zapłonu (°C)
113 °C - closed cup
Środki ochrony indywidualnej
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej