推荐产品
等級
analytical standard
品質等級
agency
EPA 1694
化驗
≥98% (TLC)
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
clinical testing
格式
neat
儲存溫度
2-8°C
SMILES 字串
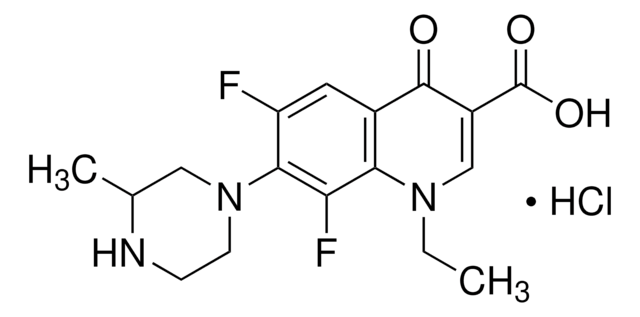
CCN1C=C(C(O)=O)C(=O)c2cc(F)c(cc12)N3CCNCC3
InChI
1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23)
InChI 密鑰
OGJPXUAPXNRGGI-UHFFFAOYSA-N
基因資訊
human ... CYP1A2(1544)
rat ... Gabra1(29705)
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
生化/生理作用
操作方式:抑制细菌 DNA 复制
抗菌谱:革兰氏阴性菌; 对革兰氏阳性菌效果较差
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
分析证书(COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
其他客户在看
商品
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门