おすすめの製品
アッセイ
≥98% (TLC)
フォーム
powder
色
white
溶解性
H2O: 20 mg/mL (with warming)
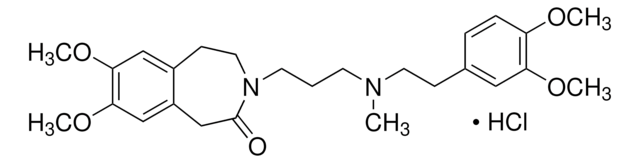
SMILES記法
Cl[H].Cl[H].C(CN1CCN(CCOC(c2ccccc2)c3ccccc3)CC1)Cc4ccccc4
InChI
1S/C28H34N2O.2ClH/c1-4-11-25(12-5-1)13-10-18-29-19-21-30(22-20-29)23-24-31-28(26-14-6-2-7-15-26)27-16-8-3-9-17-27;;/h1-9,11-12,14-17,28H,10,13,18-24H2;2*1H
InChI Key
NQWRSILGEXNJIT-UHFFFAOYSA-N
アプリケーション
GBR 12935 dihydrochloride has been used as a dopamine reuptake inhibitor to study its effects on noradrenergic stimulation in the medial prefrontal cortex of rats (mPFC). It has also been used as a selective dopamine transporter (DAT) inhibitor to study its effects on behavior and neurochemical response in enriched condition (EC) and impoverished condition (IC) rats.
生物化学的/生理学的作用
GBR 12935 inhibits the dopamine and norepinephrine transporters with Kis of 21.5 nM and 225 nM, respectively. It does not inhibit the serotonin transporter (Ki = 6.5 mM). GBR 12935 binds to a nondopaminergic piperazine site in blood platelets and brain that has been identified as cytochrome P450.
特徴および利点
This compound is featured on the Biogenic Amine Transporters page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
保管分類コード
13 - Non Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
G9659-25MG:
G9659-BULK:
G9659-100MG:
G9659-VAR:
G9659-25MG-PW:
G9659-100MG-PW:
P Allard et al.
Journal of neurochemistry, 62(1), 342-348 (1994-01-01)
The presence of multiple [3H]GBR-12935 binding sites in the human brain has been revealed in several recent studies. One site represents the dopamine uptake site. In rat brain it was demonstrated that [3H]GBR-12935 also binds to nondopaminergic "piperazine acceptor sites."
Rita Gálosi et al.
Behavioural brain research, 344, 57-64 (2018-02-18)
Effects of destroyed noradrenergic (NE) innervation in the medial prefrontal cortex (mPFC) were examined on dopamine (DA) content and metabolism. Six-hydroxy-DOPA (6-OHDOPA) or 6-hydroxy-dopamine (6-OHDA) in combination with a potent DA reuptake inhibitor GBR 12935 or 6-OHDA were injected bilaterally
I Gordon et al.
Life sciences, 55(3), 189-199 (1994-01-01)
It remains controversial whether blood platelet can be used as a peripheral model for the central presynaptic dopaminergic neurons. We investigated the existence of dopamine transport complex in human blood platelet membranes using the selective dopamine uptake inhibitor [3H]GBR 12935
Jun Zhu et al.
Behavioural brain research, 148(1-2), 107-117 (2003-12-20)
Rats raised in an enriched condition (EC) during development display increased hyperactivity to the effect of acute amphetamine compared to rats raised in an impoverished condition (IC). The present study determined whether environmental enrichment differentially alters the effects of GBR
T Hiroi et al.
Biochemical pharmacology, 53(12), 1937-1939 (1997-06-15)
The binding of [3H]1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl propyl) piperazine (GBR-12935), an antagonist of the dopamine transporter, to human P450s expressed in yeast cells was investigated. Among the ten forms of human P450 tested (CYP1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C18, 2D6, 2E1, and
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)