おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
solid
色
white
溶解性
DMSO: ≥2 mg/mL
H2O: insoluble
保管温度
2-8°C
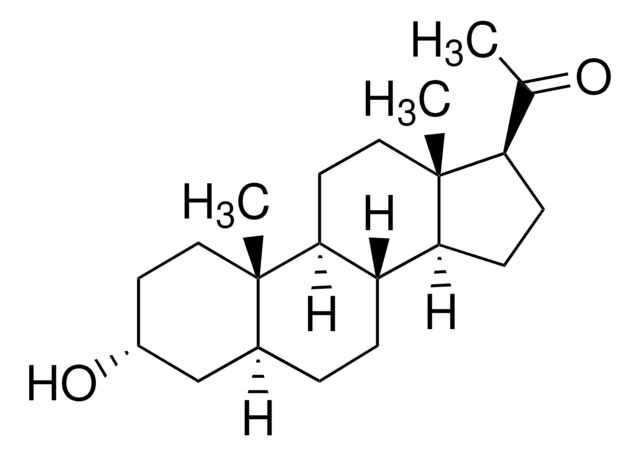
SMILES記法
[H][C@@]12CC[C@@]3([H])[C@]4([H])CC[C@H](C(C)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@](C)(O)C2
InChI
1S/C22H36O2/c1-14(23)17-7-8-18-16-6-5-15-13-20(2,24)11-12-21(15,3)19(16)9-10-22(17,18)4/h15-19,24H,5-13H2,1-4H3/t15-,16-,17+,18-,19-,20+,21-,22+/m0/s1
InChI Key
PGTVWKLGGCQMBR-FLBATMFCSA-N
遺伝子情報
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879)
mouse ... Gabrg2(14406)
生物化学的/生理学的作用
特徴および利点
品質
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
G7795-VAR:
G7795-5MG:
G7795-BULK:
G7795-IP:
G7795-SMPL:
G7795-25MG:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
資料
We offer many products related to GABAA receptors for your research needs.
お客様の研究ニーズを満たす、GABAA受容体に関連した多数の製品をご用意しています。
DISCOVER Bioactive Small Molecules for Neuroscience
関連コンテンツ
神経科学分野における低分子生理活性物質の創薬研究
アクティブなフィルタ
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)