O3139
Oxamflatin
≥98% (HPLC), solid
Sinonimo/i:
(2E)-5-[3-(Phenylsulfonylamino)phenyl]-pent-2-en-4-ynohydroxamic acid
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Saggio
≥98% (HPLC)
Stato
solid
Solubilità
DMSO: soluble 13 mg/mL
Temperatura di conservazione
2-8°C
Stringa SMILE
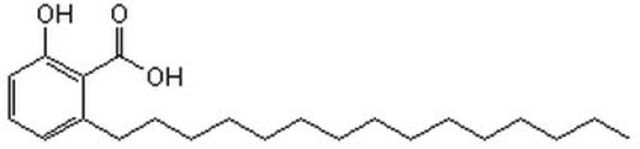
ONC(=O)\C=C\C#Cc1cccc(NS(=O)(=O)c2ccccc2)c1
InChI
1S/C17H14N2O4S/c20-17(18-21)12-5-4-7-14-8-6-9-15(13-14)19-24(22,23)16-10-2-1-3-11-16/h1-3,5-6,8-13,19,21H,(H,18,20)/b12-5+
QRPSQQUYPMFERG-LFYBBSHMSA-N
Applicazioni
- to study its effect on specificity protein 1 (Sp1) transcription and transactivation activity and CD1d mRNA expression in various tumor cells[1]
- to study its inhibitory effect on long transcript- survival motor neuron gene 2 (SMN2) silencing mediated by DNA methylation[2]
- to study its effect on somatic embryogenesis in Coffea arabica thorough a miniaturized and automated screening system[3]
- to study its stand-alone effect on cytarabine-sensitive and resistant cells.[4]
Azioni biochim/fisiol
Caratteristiche e vantaggi
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Cancer research has revealed that the classical model of carcinogenesis, a three step process consisting of initiation, promotion, and progression, is not complete.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.