A93607
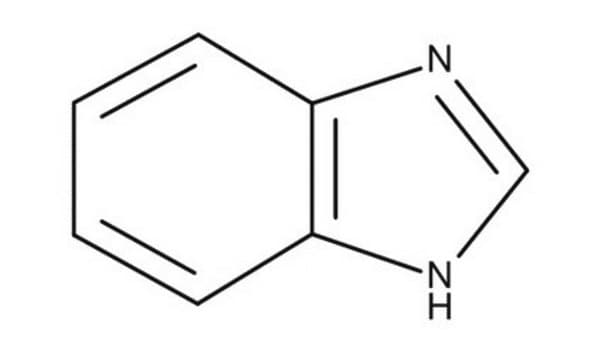
4-Azabenzimidazole
99%
Sinonimo/i:
1H-Imidazo[4,5-b]pyridine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H5N3
Numero CAS:
Peso molecolare:
119.12
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
eCl@ss:
32151902
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Forma fisica
powder
Punto di fusione
148-151 °C (lit.)
Stringa SMILE
c1cnc2nc[nH]c2c1
InChI
1S/C6H5N3/c1-2-5-6(7-3-1)9-4-8-5/h1-4H,(H,7,8,9)
GAMYYCRTACQSBR-UHFFFAOYSA-N
Categorie correlate
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
E Nicolaï et al.
Journal of medicinal chemistry, 36(9), 1175-1187 (1993-04-30)
A series of 1-benzylbenzimidazole and 3-benzylimidazo[4,5-b]pyridine substituted in the 2-position by an alkanoic or mercaptoalkanoic acid chain was synthesized for evaluation as potential thromboxane A2/prostaglandin H2 (TXA2/PGH2) receptor antagonists. The affinity of each compound for washed human platelet TXA2/PGH2 receptors
M J Wanner et al.
Nucleosides, nucleotides & nucleic acids, 23(8-9), 1313-1320 (2004-12-02)
Nitration of substituted (1-deaza)purines using a mixture of tetrabutylammonium nitrate (TBAN) and trifluoracetic acid anhydride (TFAA) was applied to prepare nitrosubstituted (1-deaza)purines at low temperature. The nitro group influences the system twofold: 1) it activates other substituents towards nucleophilic aromatic
Larissa B Krasnova et al.
The Journal of organic chemistry, 75(24), 8662-8665 (2010-11-26)
A new method for the synthesis of dihydroimidazo[1,2-a][1,3,5]triazin-4(6H)-ones via copper(I)-catalyzed hydroamination was developed. In addition, for the first time, iodoalkynes were shown to participate in the copper(I)-catalyzed intramolecular hydroamination reaction with exclusive formation of E-isomers.
Muhammad Taha et al.
Bioorganic chemistry, 65, 48-56 (2016-02-09)
6-Chloro-2-Aryl-1H-imidazo[4,5-b]pyridine derivatives 1-26 were synthesized and characterized by various spectroscopic techniques. All these derivatives were evaluated for their antiglycation, antioxidant and β-glucuronidase potential followed their docking studies. In antiglycation assay, compound 2 (IC50=240.10±2.50μM) and 4 (IC50=240.30±2.90μM) was found to be
Suresh S Pujari et al.
The Journal of organic chemistry, 75(24), 8693-8696 (2010-11-13)
Template-free cross-linking of single-stranded DNA bearing octadiynyl side chains at the 7-position of 8-aza-7-deazapurine moieties with bisfunctional azides is reported employing a Cu(I)-catalyzed azide-alkyne "bis-click" reaction. Bis-adducts were formed when the bis-azide:oligonucleotide ratio was 1:1; monofunctionalization occurred when the ratio
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![Imidazo[1,2-a]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/109/863/81ccb63f-07c6-4271-b317-1ba58979d455/640/81ccb63f-07c6-4271-b317-1ba58979d455.png)
![3-Methyl-3H-imidazo[4,5-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/309/037/0969ed2b-d1ce-49e9-87c8-ef436d41719a/640/0969ed2b-d1ce-49e9-87c8-ef436d41719a.png)



![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)


