推荐产品
等級
certified reference material
品質等級
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIB (Portugal)
濃度
1.0 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
格式
single component solution
儲存溫度
2-8°C
SMILES 字串
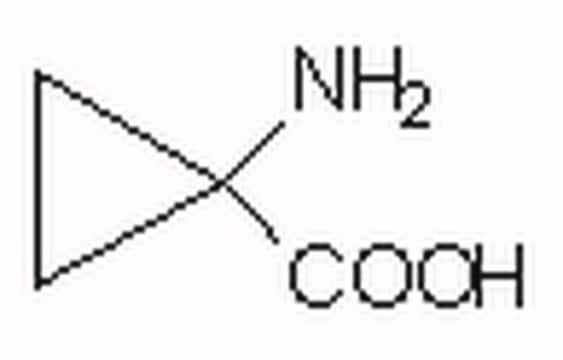
NC(C)(C)CC1=CC=CC=C1
InChI
1S/C10H15N/c1-10(2,11)8-9-6-4-3-5-7-9/h3-7H,8,11H2,1-2H3
InChI 密鑰
DHHVAGZRUROJKS-UHFFFAOYSA-N
一般說明
Snap-N-Spike®参比溶液,适用于通过LC-MS/MS或GC/MS进行临床毒理学或尿液药物检测。苯乙胺类似物芬特明是一种兴奋剂和厌食剂。该药物是已撤回的抗肥胖药fen-phen的成分。
應用
- Phentermine solution for weight loss research: Phentermine is extensively studied as an appetite suppressant in obesity treatment. Its effectiveness in weight loss regimens is often analyzed through pharmaceutical research, where the solution form facilitates precise dosing and control in clinical trials, enhancing the understanding of its pharmacodynamics and therapeutic potential (Ni et al., 2015).
法律資訊
CERILLIANT is a registered trademark of Merck KGaA, Darmstadt, Germany
Snap-N-Shoot is a registered trademark of Cerilliant Corporation
Snap-N-Spike is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
其他客户在看
Eva Manakova et al.
Neuro endocrinology letters, 33 Suppl 3, 179-182 (2013-01-29)
Both, obesity as well as anorexia may be associated with infertility and other complications of pregnancy. Weight loss during pregnancy is therefore considered a risk factor. Weight loss and appetite suppressant are contraindicated during pregnancy, but the unintended exposure is
Prescrire international, 22(136), 61-64 (2013-04-18)
The cornerstones of treatment for obesity, and even more so for simple overweight, are dietary measures and physical exercise. There are no drugs with a favourable harm-benefit balance in this setting. A fixed-dose combination of topiramate, an antiepileptic drug, and
Two new drugs approved for obesity.
Annette M Johnson
South Dakota medicine : the journal of the South Dakota State Medical Association, 65(9), 356-357 (2012-10-17)
Michael H Davidson et al.
The American journal of cardiology, 111(8), 1131-1138 (2013-02-05)
The aim of this analysis was to evaluate changes in cardiovascular risk factors in obese patients with dyslipidemia and/or hypertension receiving phentermine (PHEN) and topiramate extended-release (TPM ER). In the 56-week, randomized, double-blind, placebo-controlled, multicenter CONQUER trial, PHEN/TPM ER demonstrated
David H Winslow et al.
Sleep, 35(11), 1529-1539 (2012-11-02)
To evaluate safety and efficacy of phentermine 15 mg plus extended-release topiramate 92 mg for treatment of moderate to severe obstructive sleep apnea (OSA) in obese adults. This phase 2, randomized, double-blind, placebo-controlled study included 2-week screening and 28-week treatment
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门