所有图片(1)
About This Item
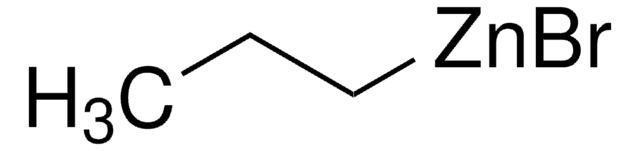
线性分子式:
CH3(CH2)3ZnBr
CAS号:
分子量:
202.41
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
濃度
0.5 M in THF
密度
0.958 g/mL at 25 °C
儲存溫度
2-8°C
SMILES 字串
CCCC[Zn]Br
InChI
1S/C4H9.BrH.Zn/c1-3-4-2;;/h1,3-4H2,2H3;1H;/q;;+1/p-1
InChI 密鑰
HMBGXQKLGHIDMN-UHFFFAOYSA-M
應用
Butylzinc bromide is an organozinc compound, which can be used as a reagent:
- In palladium-catalyzed Negishi cross-coupling reaction to construct carbon-carbon bond by reacting with organic halides or triflates.
- To prepare n-butylanisole by treating with 4-bromoanisole in the presence of a tetraphosphine ligand and a palladium catalyst.
法律資訊
Rieke® Metals, Inc. 产品 ®Rieke Metals, Inc. 的注册商标
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
標靶器官
Respiratory system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
1.4 °F - closed cup
閃點(°C)
-17 °C - closed cup
其他客户在看
Synthesis of all-cis-3-(2-diphenylphosphinoethyl)-1, 2, 4-tris (diphenylphosphinomethyl) cyclopentane (Ditricyp) from dicyclopentadiene
Kondolff I, et al.
Tetrahedron, 63(38), 9514-9521 (2007)
Room-temperature Negishi cross-coupling of unactivated alkyl bromides with alkyl organozinc reagents utilizing a Pd/N-heterocyclic carbene catalyst
Hadei N, et al.
The Journal of Organic Chemistry, 70(21), 8503-8507 (2005)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门