所有图片(1)
About This Item
经验公式(希尔记法):
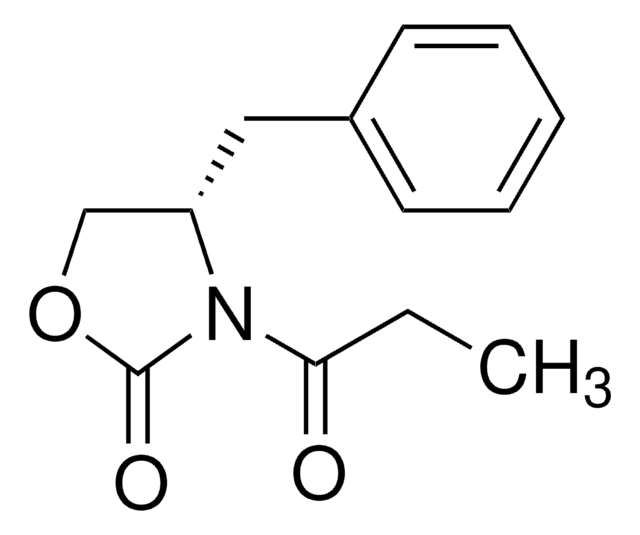
C13H15NO3
CAS号:
分子量:
233.26
MDL编号:
UNSPSC代码:
12352005
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
99%
表单
solid
旋光性
[α]20/D −102°, c = 1 in ethanol
光学纯度
ee: 99% (HPLC)
mp
44-46 °C (lit.)
官能团
phenyl
SMILES字符串
CCC(=O)N1[C@@H](COC1=O)Cc2ccccc2
InChI
1S/C13H15NO3/c1-2-12(15)14-11(9-17-13(14)16)8-10-6-4-3-5-7-10/h3-7,11H,2,8-9H2,1H3/t11-/m1/s1
InChI key
WHOBYFHKONUTMW-LLVKDONJSA-N
一般描述
(R)-(−)-4-Benzyl-3-propionyl-2-oxazolidinone is used as a building block in organic synthesis for the preparation of oxazolidinone derivatives.
应用
(R)-(−)-4-Benzyl-3-propionyl-2-oxazolidinone can be used as a building block for the preparation of methyl 3-[(S)-3-((R)-4-benzyl-2-oxooxazolidin-3-yl)-2-methyl-3-oxopropyl]benzoate by treating with strong base followed by the addition of methyl 3-bromomethyl benzoate.
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
Oxazolidinone cross-alkylation during Evans? asymmetric alkylation reaction
Fresno N, et al.
Tetrahedron, 67(47), 9104-9111 (2011)
A Synthetic Route to β-Hydroxytyrosine-Derived Tetramic Acids: Total Synthesis of the Fungal Metabolite F-14329
Bruckner, S, et al.
Chemistry?A European Journal , 23(24), 5692-5695 (2017)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持