T2327
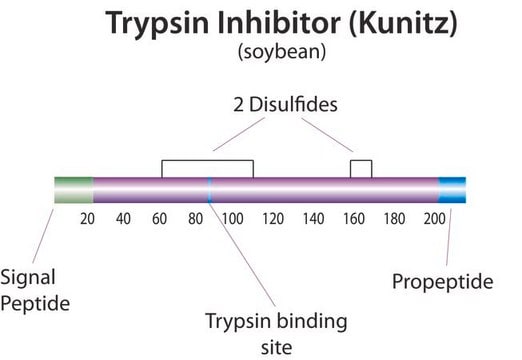
Trypsin inhibitor
lyophilized powder, ≥95% (Kunitz inhibitor, SDS-PAGE)
Synonyma:
Kunitz Inhibitor
About This Item
Doporučené produkty
Název produktu
Trypsin Inhibitor from Glycine max (soybean), BioUltra, lyophilized powder, ≥95% (Kunitz inhibitor, SDS-PAGE)
biological source
Glycine max (soybean)
product line
BioUltra
assay
≥95% (Kunitz inhibitor, SDS-PAGE)
form
lyophilized powder
storage temp.
2-8°C
Související kategorie
General description
Application
- as a standard protein to measure the amount of endogenous trypsin inhibitor present in midgut lysate (M1) of Riptortus pedestris
- as a standard to compare the trypsin inhibitory activity of the purified protein
- to monitor the trypsin inhibitory activity by fractionating in MonoS cation exchange chromatography
- as an trypsin inhibitor
Biochem/physiol Actions
Unit Definition
Preparation Note
Analysis Note
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Protokoly
This technical article described the Enzymatic Assay of Trypsin Inhibitor.
Chromatograms
application for HPLCNáš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.