SAE0046
Carboxypeptidase A from bovine pancreas
(Type II-PMSF treated), ≥20 units/mg protein
Synonyma:
Carboxypeptidase A, Carboxypolypeptidase, Peptidyl-L-amino-acid hydrolase
About This Item
Doporučené produkty
biological source
bovine pancreas
Quality Level
form
aqueous suspension
quality
(Type II-PMSF treated)
specific activity
≥20 units/mg protein
mol wt
~35 kDa
purified by
2× crystallization
impurities
≤0.05 BTEE units/mg protein chymotrpsin
≤10 BAEE units/mg protein trypsin
storage temp.
2-8°C
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
Biochem/physiol Actions
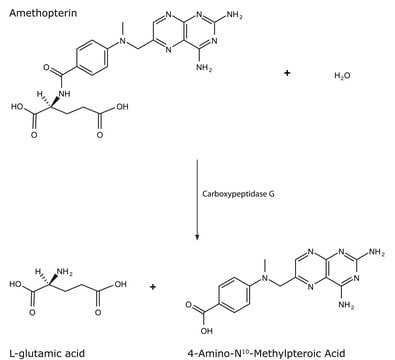
Unit Definition
Preparation Note
Analysis Note
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.