PHR1782
Hydroxychloroquine Sulfate
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyma:
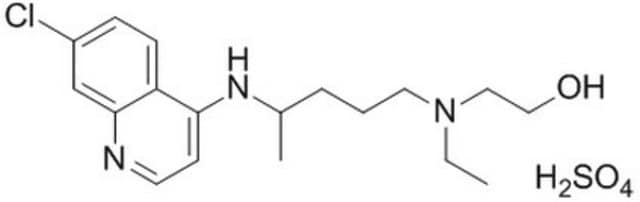
Hydroxychloroquine sulfate, 7-Chloro-4-[4-(N-ethyl-N-b-hydroxyethylamino)-1-methylbutylamino]quinoline sulfate
About This Item
Doporučené produkty
biological source
synthetic
Quality Level
grade
certified reference material
pharmaceutical secondary standard
agency
BP
EP
USP
traceable to BP 1100
traceable to USP 1327000
API family
hydroxychloroquine
form
powder or crystals
CofA
current certificate can be downloaded
packaging
pkg of 1 g
storage condition
protect from light
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
color
white to off-white
pH
3.5-5.5 (1% in aqueous solution, pH <6.0)
mp
238-241 °C
solubility
chloroform: practically insoluble
ethanol: practically insoluble
ether: practically insoluble
water: soluble
application(s)
pharmaceutical (small molecule)
shipped in
ambient
storage temp.
2-30°C
SMILES string
OS(O)(=O)=O.CCN(CCO)CCCC(C)Nc1ccnc2cc(Cl)ccc12
InChI
1S/C18H26ClN3O.H2O4S/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18;1-5(2,3)4/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21);(H2,1,2,3,4)
InChI key
JCBIVZZPXRZKTI-UHFFFAOYSA-N
Gene Information
human ... TLR7(51284) , TLR9(54106)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Biochem/physiol Actions
Analysis Note
Other Notes
Footnote
related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.