26454
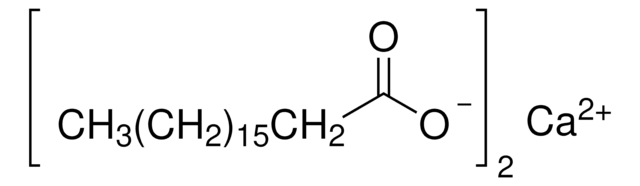
Magnesium stearate
puriss., meets analytical specification of Ph. Eur., BP, ≥90% stearic and palmitic acid basis, ≥40% stearic acid basis (GC), 4.0-5.0% Mg basis (calc on dry sub.)
Synonyma:
Stearic acid magnesium salt
About This Item
Doporučené produkty
grade
puriss.
assay
≥40% stearic acid basis (GC)
≥90% stearic and palmitic acid basis
4.0-5.0% Mg basis (calc on dry sub.)
quality
meets analytical specification of Ph. Eur., BP
impurities
acidity or alkalinity, complies
microbiological impurity, in accordance
residual solvents, complies
loss
≤6.0% loss on drying, 105 °C
mp
200 °C (lit.)
acid value
195‑210(fatty acid)
solubility
alcohol: insoluble
diethyl ether: insoluble
water: insoluble
anion traces
chloride (Cl-): ≤1000 mg/kg
sulfate (SO42-): ≤5000 mg/kg
cation traces
Cd: ≤3 mg/kg
Ni: ≤5 mg/kg
Pb: ≤10 mg/kg
suitability
in accordance for fatty acid composition
passes test for identity
application(s)
pharmaceutical (small molecule)
SMILES string
CCCCCCCCCCCCCCCCCC(=O)O[Mg]OC(=O)CCCCCCCCCCCCCCCCC
InChI
1S/2C18H36O2.Mg/c2*1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20;/h2*2-17H2,1H3,(H,19,20);/q;;+2/p-2
InChI key
HQKMJHAJHXVSDF-UHFFFAOYSA-L
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Application
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.