234923
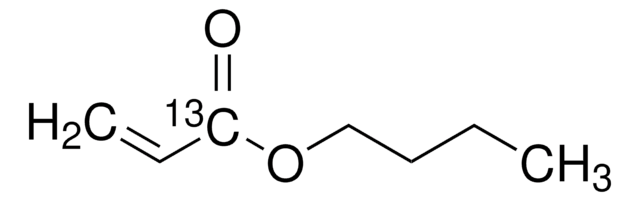
Butyl acrylate
≥99%, contains 10-60 ppm monomethyl ether hydroquinone as inhibitor
Synonyma:
n-Butyl acrylate
About This Item
Doporučené produkty
vapor density
>1 (vs air)
Quality Level
vapor pressure
3.3 mmHg ( 20 °C)
assay
≥99%
form
liquid
autoignition temp.
559 °F
contains
10-60 ppm monomethyl ether hydroquinone as inhibitor
expl. lim.
9.9 %
refractive index
n20/D 1.418 (lit.)
bp
145 °C (lit.)
density
0.894 g/mL at 25 °C (lit.)
SMILES string
CCCCOC(=O)C=C
InChI
1S/C7H12O2/c1-3-5-6-9-7(8)4-2/h4H,2-3,5-6H2,1H3
InChI key
CQEYYJKEWSMYFG-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Související kategorie
General description
Butyl acrylate undergoes radical copolymerization with benzoxazine containing a vinyl group to afford copolymers. Heck coupling reactions of aryl bromides with n-butyl acrylate mediated by phosphine-imidazolium salt have been reported. Copolymerization of styrene and n-butyl acrylate by ATRP catalyzed by CuBr/4,4′-di(5-nonyl)-2,2′-bipyridine has been described.
Application
- An electrolyte additive in lithium-ion batteries to improve their low-temperature performance. The addition of BA to the electrolyte led to a significant improvement in the low-temperature performance of the battery, including enhanced ionic conductivity and improved rate capability.
- A monomer to synthesize a shape memory polymer network that contains magnetic nanoparticles for various applications, including actuators and biomedical devices.
- A monomer for the preparation of a polymeric semiconductor with intrinsically stretchable properties. This polymer material is used as a component in field-effect transistor applications.
- Poly(butyl acrylate) particles.
- Poly(butyl acrylate-b-acrylic acid) block copolymer.
- Amphiphilic charged diblock copolymers poly(butyl acrylate)-b-poly(acrylic acid).
- Poly(n-butyl acrylate), via atom transfer radical polymerization (ATRP) of n-butyl acrylate in the presence of CuIBr/4,4′-di(5-nonyl)-2,2′-bipyridine (catalyst).
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
98.6 °F - closed cup
flash_point_c
37 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Sortimentní položky
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.