126535
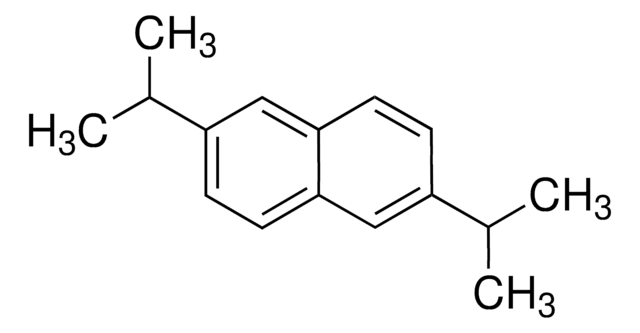
2,6-Dimethylnaphthalene
99%
Synonyma:
2,6-Dimethylnaphthalene
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Lineární vzorec:
C10H6(CH3)2
Číslo CAS:
Molekulová hmotnost:
156.22
Beilstein/REAXYS Number:
1903544
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
assay
99%
bp
262 °C (lit.)
mp
106-110 °C (lit.)
SMILES string
Cc1ccc2cc(C)ccc2c1
InChI
1S/C12H12/c1-9-3-5-12-8-10(2)4-6-11(12)7-9/h3-8H,1-2H3
InChI key
YGYNBBAUIYTWBF-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
2,6-dimethylnaphthalene is a polycyclic aromatic hydrocarbon available in the water bodies and can be determined by gas chromatography with flame-ionization.
Application
2,6-Dimethylnaphthalene hs been used as a substrate in intramolecular isotope effect experiments to compare substrate dynamics in CYP2E1 and CYP2A6.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
N Miyachi et al.
Applied and environmental microbiology, 59(5), 1504-1506 (1993-05-01)
Three bacterial strains, identified as Alcaligenes sp. strain D-59 and Pseudomonas sp. strains D-87 and D-186, capable of growing on 2,6-dimethylnaphthalene (2,6-DMN) as the sole source of carbon and energy were isolated from soil samples. 2,6-Naphthalene dicarboxylic acid was formed
Dietary accumulation of dimethylnaphthalene by the grass shrimp Palaemonetes pugio under stable and fluctuating temperatures.
T M Dillon
Bulletin of environmental contamination and toxicology, 28(2), 149-153 (1982-02-01)
Effect of Aroclor 1254 on the biological fate of 2,6-dimethylnaphthalene in coho salmon (Oncorhynchus kisutch).
T K Collier et al.
Bulletin of environmental contamination and toxicology, 34(1), 114-120 (1985-01-01)
Aryl sulfate formation in sea urchins (Strongylocentrotus droebachiensis) ingesting marine algae (Fucus distichus) containing 2,6-dimethylnaphthalene.
D C Malins et al.
Environmental research, 27(2), 290-297 (1982-04-01)
Z A Shamsuddin et al.
Drug metabolism and disposition: the biological fate of chemicals, 14(6), 724-732 (1986-11-01)
Metabolism of the environmental contaminant 2,6-dimethylnaphthalene (2,6-DMN) by rat liver microsomes and an NADPH-regenerating system led to the formation of three ring oxidation metabolites--2,6-dimethyl-3-naphthol, 2,6-dimethyl-3,4-naphthoquinone, and 3,4-dihydro-3,4-dihydroxy-2,6-dimethylnaphthalene--and one side chain oxidation metabolite--2-hydroxymethyl-6-methylnaphthalene. In addition, one metabolite remained unidentified. Pretreatment of
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.