230367
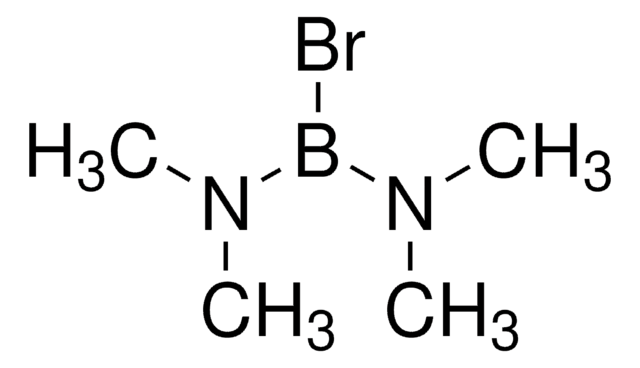
Boron tribromide
≥99.99%
Sinónimos:
Tribromoboron
About This Item
Productos recomendados
vapor density
8.6 (vs air)
Quality Level
vapor pressure
40 mmHg ( 14 °C)
assay
≥99.99%
form
liquid
bp
~90 °C (lit.)
mp
−46 °C (lit.)
density
2.60 g/mL at 20 °C (lit.)
SMILES string
BrB(Br)Br
InChI
1S/BBr3/c2-1(3)4
InChI key
ILAHWRKJUDSMFH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Drug intermediate 6-nitro-L-DOPA
- Luminescent polystyrene derivatives with sterically protected carbazolylborane moieties
- High-quality boron-doped graphene via Wurtz-type reductive coupling reaction
- Mercapto-(+)-methamphetamine haptens for synthesis of (+)-methamphetamine conjugate vaccines with improved epitope densities
- Micrometer-sized organic molecule-DNA hybrid structures
- Borane complexes via electrophilic aromatic borylation reactions
- A 5-HT2C receptor agonist
- Biphenyl-derivatives possessing tertiary amino groups as β-secretase(BACE1) inhibitors for the treatment of Alzheimer′s disease
- A highly near-IR region fluorescent p-extended boron aza-dipyrromethene moiety unit
- Tetrahydroisoquinoline derivatives via intramolecular cyclization of methoxy-substituted N-phenethylimides
For use with
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico