167134
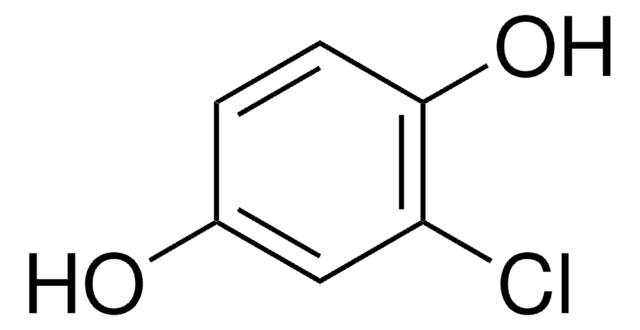
Bromohydroquinone
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
BrC6H3(OH)2
Número de CAS:
Peso molecular:
189.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
mp
112-116 °C (lit.)
functional group
bromo
SMILES string
Oc1ccc(O)c(Br)c1
InChI
1S/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
InChI key
REFDOIWRJDGBHY-UHFFFAOYSA-N
Categorías relacionadas
Application
Bromohydroquinone was used in the synthesis of Π-conjugated polymers composed of alkyl carbazole/dialkoxyphenylene and squaraine units via Sonogashira cross-coupling reactions. It was used in the preparation of 2-bromobenzoquinone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
S S Lau et al.
The Journal of pharmacology and experimental therapeutics, 230(2), 360-366 (1984-08-01)
2-Bromohydroquinone was identified as a metabolite of both bromobenzene and o-bromophenol in the rat in vivo and in vitro. Identification was based on high-pressure liquid chromatography and gas chromatography-mass spectrometry. Formation of 2-bromohydroquinone by rat liver microsomes from both bromobenzene
S S Lau et al.
Drug metabolism and disposition: the biological fate of chemicals, 15(6), 801-807 (1987-11-01)
Homogenates from rat renal papillae, a rich source of the prostaglandin (PG) H synthase system (PHS), metabolized [14C]2-bromohydroquinone, in the presence of arachidonic acid, to products which are covalently bound to protein. The co-oxidation of 2-bromohydroquinone caused a concentration-dependent stimulation
T J Monks et al.
Toxicology and applied pharmacology, 103(3), 557-563 (1990-05-01)
Glutathione (GSH) conjugates of 2-bromohydroquinone are more difficult to oxidize than the parent hydroquinone. Hydrolysis catalyzed by gamma-glutamyl transpeptidase (gamma-GT), however, results in the formation of the corresponding cysteine conjugate which is more readily oxidized than the parent hydroquinone. N-Acetylation
S S Lau et al.
Toxicology and applied pharmacology, 103(1), 121-132 (1990-03-15)
We have previously shown that the renal necrosis observed after 2-bromohydroquinone (2-BrHQ) administration to rats is probably caused by the formation of 2-Br-(diglutathion-S-yl)HQ (2-Br-[diGSyl]HQ), since injection of this conjugate caused severe proximal tubular necrosis. In the present study we report
Hetero Diels-Alder Reactions of 1-Acetylamino-and 1-Dimethylamino-1-azadienes with Benzoquinones.
Perez JM, et al.
Tetrahedron, 56(11), 1561-1567 (2000)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico