E7521
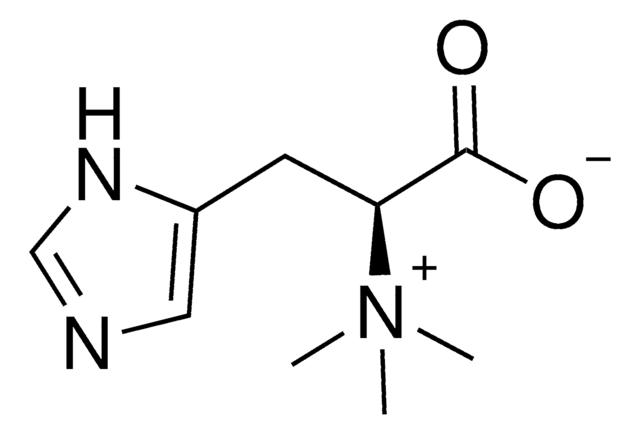
L-(+)-Ergothioneine
Sinónimos:
2-mercaptohistidine trimethyl betaine, Ergothioneine, Sympectothion, Thiasine, Thiolhistidine-betaine, Thioneine, (S)-α-Carboxy-N,N,N-trimethyl-2-mercapto-1H-imidazole-4-ethanaminium inner salt
About This Item
Productos recomendados
biological source
fungus (Actinomycetales)
fungus (Ascomycota)
fungus (Basidiomycota)
Quality Level
assay
≥98.0%
form
powder
mol wt
229.30
storage condition
(Keep container tightly closed in a dry and well-ventilated place)
technique(s)
protein quantification: suitable
solubility
water: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
C[N+](C)(C)[C@@H](Cc1c[nH]c(S)n1)C([O-])=O
InChI
1S/C9H15N3O2S/c1-12(2,3)7(8(13)14)4-6-5-10-9(15)11-6/h5,7H,4H2,1-3H3,(H2-,10,11,13,14,15)/t7-/m0/s1
InChI key
SSISHJJTAXXQAX-ZETCQYMHSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Research area: Apoptosis
Application
L-(+)-Ergothioneine has been used:
- as a component of the maturation medium for cumulus-oocyte complexes (COCs) to test protective function on lipid peroxide formation
- as an antioxidant compound to test type 2 diabetes patients
- as a positive control in solute carrier protein 22 A4 (SLC22A4) transport assay
Biochem/physiol Actions
Packaging
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico