N7764
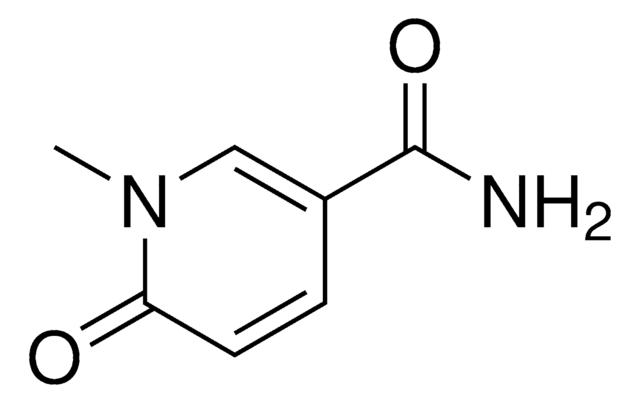
Nicotinic acid mononucleotide
Sinonimo/i:
Nicotinate mononucleotide
About This Item
Prodotti consigliati
Origine biologica
animal
Livello qualitativo
Saggio
≥98.0% (HPLC)
Stato
powder
tecniche
HPLC: suitable
Colore
white
Temperatura di conservazione
−20°C
Stringa SMILE
OC1C(O)C(OC1COP(O)(O)=O)[N]2=CC(=CC=C2)C(O)=O
InChI
1S/C11H15NO9P/c13-8-7(5-20-22(17,18)19)21-10(9(8)14)12-3-1-2-6(4-12)11(15)16/h1-4,7-10,13-14H,5H2,(H,15,16)(H2,17,18,19)
IUJWGLOJJKFASX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.