This product is soluble in DMSO (10 mg/mL). At higher concentrations, heating is required for the product to dissolve completely (approximately 10 minutes at 95°C), but some precipitation occurs upon cooling to room temperature. It is also soluble in 1 N NaOH (50 mg/mL) and methanol (50 mg/mL).
C9911
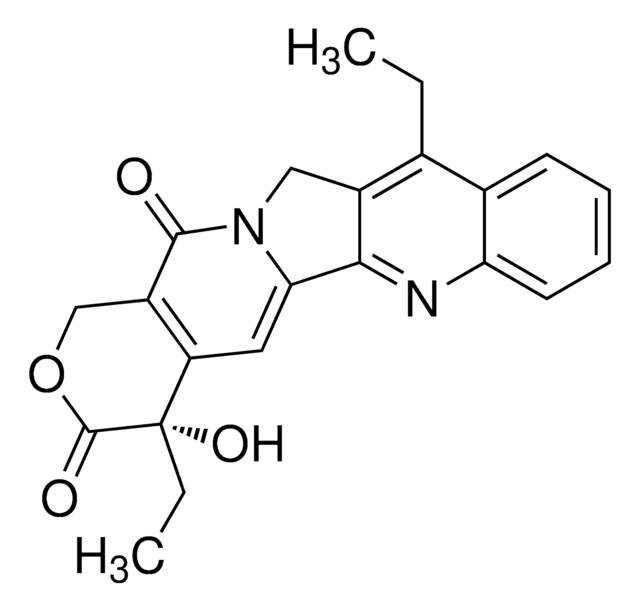
(S)-(+)-Camptothecin
≥90% (HPLC), powder, DNA topoisomerase I inhibitor
Sinonimo/i:
(S)-4-Ethyl-4-hydroxy-1H-pyrano-[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Nome del prodotto
(S)-(+)-Camptothecin, ≥90% (HPLC), powder
Livello qualitativo
Saggio
≥90% (HPLC)
Stato
powder
Punto di fusione
260 °C (dec.) (lit.)
Solubilità
chloroform/methanol (4:1): 5 mg/mL
Spettro attività antibiotica
neoplastics
Modalità d’azione
DNA synthesis | interferes
enzyme | inhibits
Temperatura di conservazione
2-8°C
Stringa SMILE
CC[C@@]1(O)C(=O)OCC2=C1C=C3N(Cc4cc5ccccc5nc34)C2=O
InChI
1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
VSJKWCGYPAHWDS-FQEVSTJZSA-N
Informazioni sul gene
human ... TOP1(7150)
mouse ... Prkca(18750)
rat ... Sstr2(54305)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
Caratteristiche e vantaggi
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Muta. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
We presents an article on Autophagy in Cancer Promotes Therapeutic Resistance
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
-
What is Product C9911, (S)-(+)-Camptothecin, soluble in?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the extinction coefficient of Product C9911, (S)-(+)-Camptothecin?
1 answer-
The millimolar extinction coefficients (EmM) at various wavelengths are 37.3 (220 nm); 29.2 (254 nm); 4.9 (290 nm); 19.9 (370 nm).
Helpful?
-
-
What is the solution stability of Product C9911, (S)-(+)-Camptothecin?
1 answer-
Stock solutions of camptothecin in DMSO can be stored at -20°C for up to 3 months.
Helpful?
-
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.