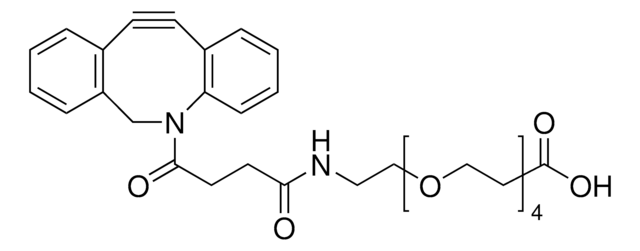
764019
Dibenzocyclooctyne-PEG4-N-hydroxysuccinimidyl ester
≥90%
Sinonimo/i:
DBCO-PEG4-NHS ester, DBCO-PEG4-SE, DBCO-PEG4-succinimidyl ester
About This Item
Prodotti consigliati
Saggio
≥90%
Forma fisica
paste
Impiego in reazioni chimiche
reaction type: click chemistry
reagent type: linker
Gruppo funzionale
NHS ester
Temperatura di conservazione
−20°C
Stringa SMILE
O=C(CCC(NCCOCCOCCOCCOCCC(ON1C(CCC1=O)=O)=O)=O)N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4
InChI
1S/C34H39N3O10/c38-30(11-12-31(39)36-25-28-7-2-1-5-26(28)9-10-27-6-3-4-8-29(27)36)35-16-18-44-20-22-46-24-23-45-21-19-43-17-15-34(42)47-37-32(40)13-14-33(37)41/h1-8H,11-25H2,(H,35,38)
RRCXYKNJTKJNTD-UHFFFAOYSA-N
Applicazioni
Applications Include:
- Protein-peptide conjugates
- Antibody-enzyme or antibody-drug conjugates
- Protein or peptide-oligonucleotide conjugates
- Surface modification
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.









![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)

![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)