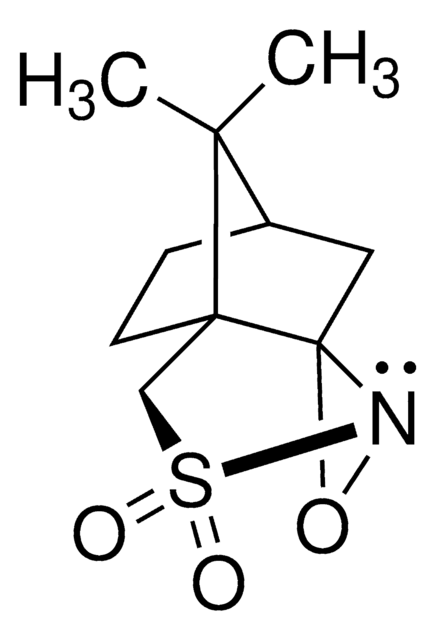
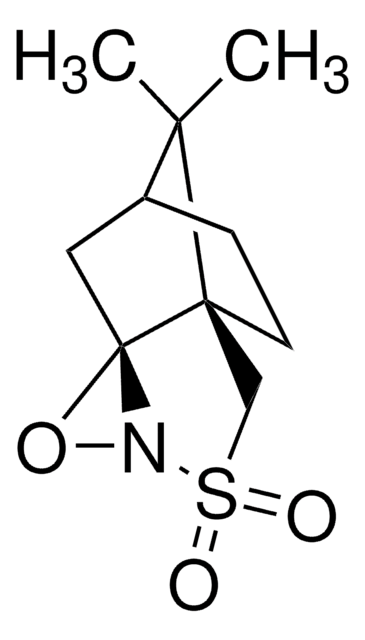
900624
Di-t-butyl oxaziridine
≥95%
Synonym(e):
Kurti oxaziridine
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
Form
liquid
Verfügbarkeit
available only in USA
Brechungsindex
n/D 1.4453
Dichte
0.90 g/mL
Lagertemp.
2-8°C
SMILES String
CC(C)(C)C1(NO1)C(C)(C)C
Anwendung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Self-react. C
Lagerklassenschlüssel
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flammpunkt (°F)
143.6 °F
Flammpunkt (°C)
62 °C
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Verwandter Inhalt
Amines and their derivatives are ubiquitous substances since they make up the overwhelming majority of drug molecules, agrochemicals as well as many compounds that are produced by plants and living organisms (i.e., natural products).
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.