278424
Poly(vinylsulfonic acid, sodium salt) solution
30-40 wt. % in H2O, technical grade
Synonyme(s) :
PVSA
About This Item
Produits recommandés
Qualité
technical grade
Niveau de qualité
Forme
liquid
Concentration
30-40 wt. % in H2O
Indice de réfraction
n20/D 1.389
Densité
1.267 g/mL at 25 °C
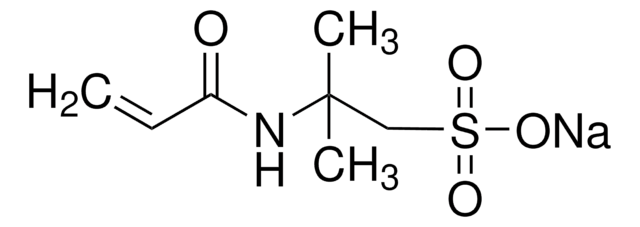
Chaîne SMILES
[Na]OS(=O)(=O)C=C
InChI
1S/C2H4O3S.Na/c1-2-6(3,4)5;/h2H,1H2,(H,3,4,5);/q;+1/p-1
Clé InChI
BWYYYTVSBPRQCN-UHFFFAOYSA-M
Application
- In the preparation of superabsorbent semi-IPN (interpenetrating polymer network) hydrogel.
- As a solid electrolyte for proton conduction in the fabrication of an all-solid-supercapacitor.
- As a crystallization controlling agent in the preparation of high-quality crystals of porous coordination polymers(CP). PVSA regulates not only the size and structure of the crystals but also their preference orientation, resulting in CP channel alignment in the bulk powdery state.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Recently, layer-by-layer (LbL) assembly has emerged as a versatile, gentle and, simple method for immobilization of functional molecules in an easily controllable thin film morphology.1,2 In this short review, we introduce recent advances in functional systems fabricated by using the mild, yet adaptable LbL technique.
We present an article that discusses two applications in particular; first, using these layers as polyelectrolyte membranes to control permeability.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique