SML2706
Jadomycin B
≥98% (HPLC)
Sinônimo(s):
1-(sec-butyl)-12-((4,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-7-hydroxy-5-methyl-8H-benzo[b]oxazolo[3,2-f]phenanthridine-2,8,13(1H,3aH)-trione
About This Item
Produtos recomendados
fonte biológica
Streptomyces venezuelae
Nível de qualidade
Ensaio
≥98% (HPLC)
Formulário
powder
condição de armazenamento
protect from light
solubilidade
DMSO: 1 mg/mL
temperatura de armazenamento
−20°C
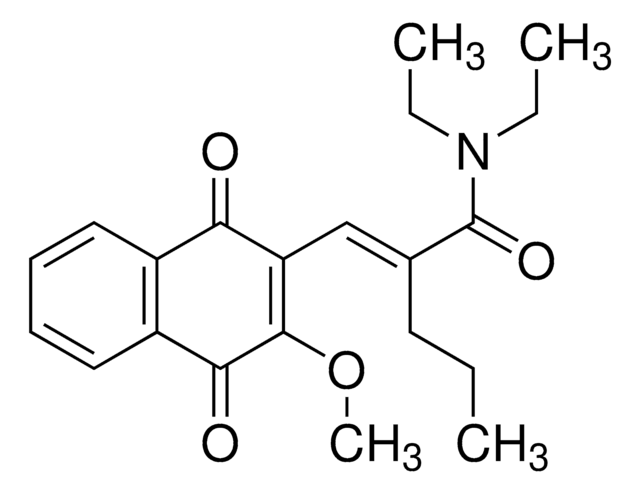
cadeia de caracteres SMILES
N21C(OC(=O)C2C(CC)C)c3c(c(cc(c3)C)O)C4=C1C(=O)c5c(cccc5OC6OC(C(C(C6)O)O)C)C4=O
InChI
1S/C30H31NO9/c1-5-13(3)24-30(37)40-29-16-9-12(2)10-17(32)21(16)23-25(31(24)29)28(36)22-15(27(23)35)7-6-8-19(22)39-20-11-18(33)26(34)14(4)38-20/h6-10,13-14,18,20,24,26,29,32-34H,5,11H2,1-4H3
chave InChI
BSBSCJRAEMDCHC-UHFFFAOYSA-N
Descrição geral
Jadomycin B displays antimicrobial, anti-tumor, aurora-B kinase inhibition, DNA cleaving and more activities.3,4,5,6
Jadomycin B was found to be active against a variety of staphylococci, including methicillin-resistant Staphylococcus aureus in a MIC of 1μg/ml.3 In addition, its anti-tumor activity was demonstrated as it kills drug-sensitive and multidrug-resistant breast cancer cell, through inhibition of type II topoisomerases and the induction of DNA damage and apoptosis. Jadomycin B (15 mM), 24-hour treatment significantly lowered the levels of topoisomerase IIa protein versus the vehicle control.4
It was also shown that Jadomycin B inhibits Aurora-B kinase activity by phosphorylation of histone H3 on Ser10 in a dose-dependent manner (10μg /mL Jadomycin B reduced H3 phosphorylation by 70%).5
Jadomycin B was also found to cleave DNA in the presence of Cu (II) by reducing it to Cu(I) which can further react with H2O2 to form hydroxyl radicals that causes DNA strand scission without the requirement of any external reducing agent. The EC50 value of Jadomycin B for single-strand scission was approximately 1.7μM.6
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)