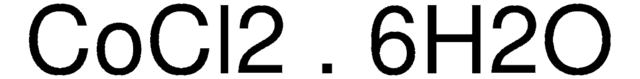255599
Cobalt(II) chloride hexahydrate
ACS reagent, 98%
Sinônimo(s):
Cobaltous chloride hexahydrate
About This Item
Produtos recomendados
grau
ACS reagent
pressão de vapor
40 mmHg ( 0 °C)
Ensaio
98%
98.0-102.0% (ACS specification)
forma
powder, crystals or chunks
adequação da reação
reagent type: catalyst
core: cobalt
Impurezas
≤0.01% insolubles
traços de ânion
nitrate (NO3-): ≤0.01%
sulfate (SO42-): ≤0.01%
traços de cátion
Ca: ≤0.005%
Cu: ≤0.002%
Fe: ≤0.005%
K: ≤0.01%
Mg: ≤0.005%
Na: ≤0.05%
Ni: ≤0.1%
Zn: ≤0.03%
cadeia de caracteres SMILES
O.O.O.O.O.O.Cl[Co]Cl
InChI
1S/2ClH.Co.6H2O/h2*1H;;6*1H2/q;;+2;;;;;;/p-2
chave InChI
GFHNAMRJFCEERV-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Dioxygenation of styrenes with N-hydroxyphthalimide to synthesize corresponding alcohols and ketones.
- Oxidative coupling of primary amines to imines.
- Friedel-Crafts reaction between indoles and activated olefins to synthesize β-indolylketones.
- Synthesis of β-enaminones and β-enamino esters.
- Reaction of acyl chlorides with diselenides or disulfides to synthesize selenol and thiol esters.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Código de classe de armazenamento
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica