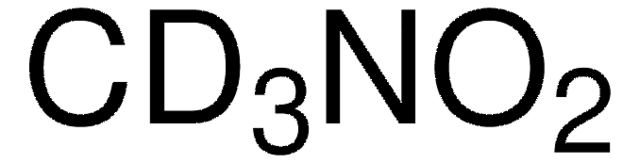230731
Nitromethane
ReagentPlus®, ≥99.0%
Sinônimo(s):
Nitrocarbol
About This Item
Produtos recomendados
densidade de vapor
2.1 (vs air)
pressão de vapor
2.7 mmHg
linha de produto
ReagentPlus®
Ensaio
≥99.0%
forma
liquid
temperatura de autoignição
784 °F
Lim. expl.
7.3 %, 33 °F
índice de refração
n20/D 1.382 (lit.)
pH
6.4 (20 °C, 0.01 g/L)
pb
101.2 °C (lit.)
pf
−29 °C (lit.)
densidade
1.127 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
chave InChI
LYGJENNIWJXYER-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- As a reagent in the synthesis of 3-nitroindoles by arylation with N-(2-bromoaryl) imidates using palladium catalyst.
- As a cyanating reagent for the synthesis of thiocyanates in presence of base (KOAc) and iodine.
- In the preparation of cobalt cage complexes from polyamines and formaldehyde.
- As a solvent in the preparation of β-substituted γ-nitroaldehydes by enantioselective cross-coupling of β-arylated or γ,δ-unsaturated aldehydes using oxidizing agent DDQ (2,3-dichloro-5,6-dicyanoquinone) and catalyst diphenylprolinol silyl ether.
Características e benefícios
- Low toxicity.
- High specific impulse.
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
Código de classe de armazenamento
4.1A - Other explosive hazardous materials
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
95.0 °F - closed cup
Ponto de fulgor (°C)
35 °C - closed cup
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica