Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
PHR1257
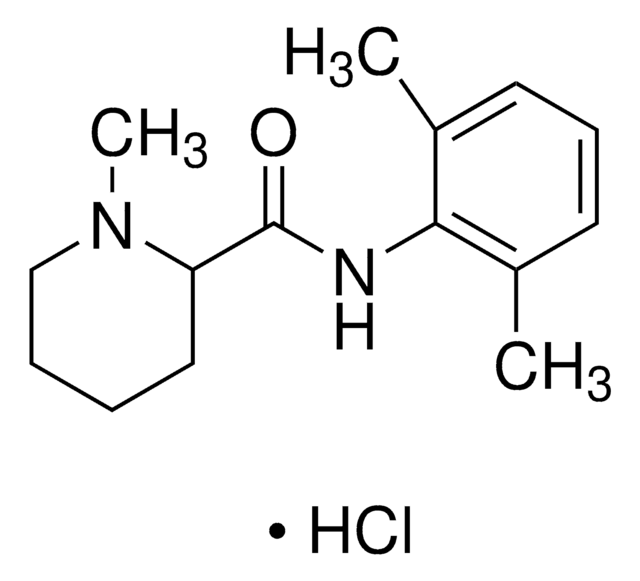
Lidocaína
Pharmaceutical Secondary Standard; Certified Reference Material
Sinônimo(s):
2-Dietilamino-N-(2,6-dimetilfenil)acetamida, Lignocaína, Xilocaína
About This Item
Produtos recomendados
grau
certified reference material
pharmaceutical secondary standard
Nível de qualidade
Agency
traceable to BP 214
traceable to Ph. Eur. L0600000
traceable to USP 1366013
família API
lidocaine
Certificado de análise (CofA)
current certificate can be downloaded
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
aplicação(ões)
pharmaceutical (small molecule)
Formato
neat
temperatura de armazenamento
2-30°C
cadeia de caracteres SMILES
Cl[H].[H]O[H].CCN(CC)CC(=O)Nc1c(C)cccc1C
InChI
1S/C14H22N2O.ClH.H2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H;1H2
chave InChI
YECIFGHRMFEPJK-UHFFFAOYSA-N
Informações sobre genes
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Lidocaine hydrochloride is an amide type local anaesthetic that belongs to the class of 1b antiarrhythmics. It is used for regional nerve blocks and infiltrative administration of anaesthesia. It prevents the entry of sodium ions into nerve endings, at the site of pain, and disrupts the electrical signal from reaching the brain.[1]
Aplicação
- Sensitive determination of lidocaine hydrochloride and cetylpyridinium chloride in their binary mixtures in different pharmaceutical formulations by three spectrophotometric-based methods
- Simultaneous analysis of aminoacridine hydrochloride and lidocaine hydrochloride in bulk powder and pharmaceutical formulation by high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC)-densitometric methods[1]
- Quantification of ceftriaxone sodium and lidocaine HCl in human plasma samples by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS)
- Quantitative analysis of lidocaine hydrochloride and miramistin in a wound healing gel sample by high-performance liquid chromatography (HPLC) combined with UV detection[2]
- Development of a method based on liquid–liquid extraction of nifedipine and lidocaine hydrochloride from human plasma samples for their subsequent analysis by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)
- Dispersive liquid-liquid microextraction (DLLME) followed by attenuated total reflectance-Fourier transform infrared measurement of dry films for the determination of lidocaine hydrochloride in human urine sample[3]
Ações bioquímicas/fisiológicas
Nota de análise
Nota de rodapé
Produtos recomendados
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica