PHR1669
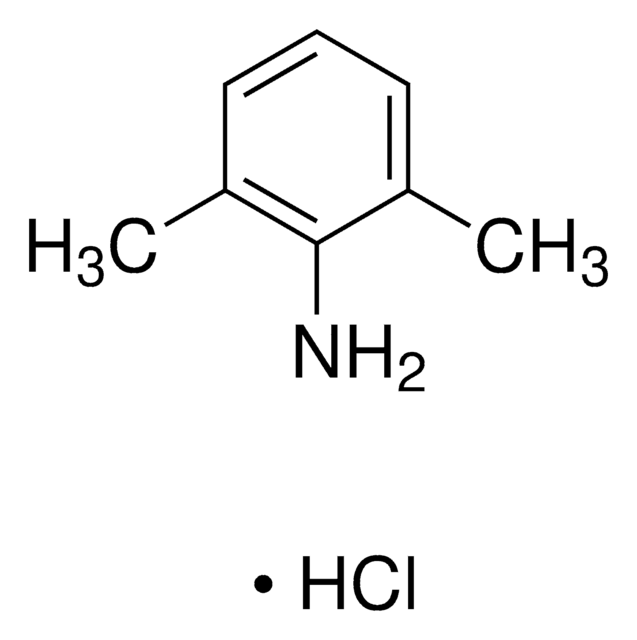
Lidocaine Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Sinônimo(s):
2,6-Dimethylaniline, Lidocaine Impurity A; 2,6 DMA, 2,6-Xylidine, 2-Amino-1,3-dimethylbenzene, 2-Amino-m-xylene
About This Item
Produtos recomendados
grau
certified reference material
pharmaceutical secondary standard
Nível de qualidade
Agency
traceable to Ph. Eur. Y0001575
pressão de vapor
<0.01 mmHg ( 20 °C)
família API
lidocaine
Certificado de análise (CofA)
current certificate can be downloaded
embalagem
pkg of 100 mg
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
índice de refração
n20/D 1.560 (lit.)
pb
214 °C/739 mmHg (lit.)
pf
10-12 °C (lit.)
densidade
0.984 g/mL at 25 °C (lit.)
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
temperatura de armazenamento
2-30°C
cadeia de caracteres SMILES
Cc1cccc(C)c1N
InChI
1S/C8H11N/c1-6-4-3-5-7(2)8(6)9/h3-5H,9H2,1-2H3
chave InChI
UFFBMTHBGFGIHF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
The standard is a certified reference material (CRM) qualified with instruments validated according to good manufacturing practices (GMP) using pharmacopeia monograph methods. It is supplied with a comprehensive certificate containing information on traceability assay results, certified purity, homogeneity tests, uncertainty statement, and stability assessment.
Lidocaine Related Compound A is a primary aromatic amine and a major metabolite of the anesthetic lidocaine. It is used as a starting material in the manufacturing of various anesthetics like lidocaine, bupivacaine, mepivacaine, etidocaine, ropivacaine, pyrrocaine, and xylazine.
Aplicação
This pharmaceutical secondary standard can also be used as follows:
- Development of an impurity selective reverse phase-high performance liquid chromatography (RP-HPLC) method to determine dexpanthenol, lidocaine hydrochloride, mepyramine maleate, and their related substances in topical dosage forms
- Testing a selective high-performance liquid chromatography-diode array detection (HPLC-DAD) method, developed for the simultaneous analysis of miconazole nitrate and lidocaine hydrochloride in their combined oral gel dosage form, for its stability-indicating properties
- Evaluation of a high-performance liquid chromatography-diode array detection (HPLC-DAD) procedure― for its stability indicating properties, developed to determine nitrofurazone and lidocaine hydrochloride in their combined dosage form
- Separation of 2,6-Dimethylaniline, its isomeric impurities, and other related impurities by isocratic and reverse-phase ultra-performance liquid chromatographic (UPLC) method
- analyze a binary mixture of lidocaine hydrochloride and cetylpyridinium chloride in presence of lidocaine impurity A by spectrophotometric methods
- determine lidocaine hydrochloride-related substance by analytical methods in pharmaceutical dosage forms
Nota de análise
Nota de rodapé
Produtos recomendados
produto relacionado
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
195.8 °F - closed cup
Ponto de fulgor (°C)
91 °C - closed cup
Choose from one of the most recent versions:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica