8.52373
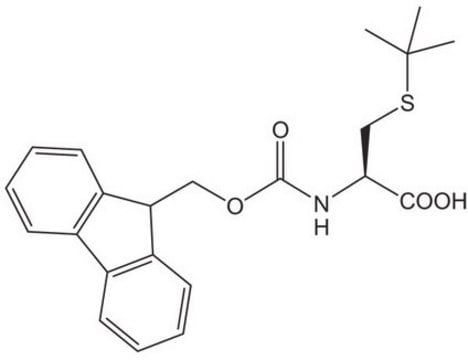
Fmoc-Cys(STmp)-OH
for peptide synthesis, Novabiochem®
Sinônimo(s):
Fmoc-Cys(STmp)-OH, N-α-Fmoc-S-2,4,6-trimethoxyphenylthio-L-cysteine
About This Item
Produtos recomendados
product name
Fmoc-Cys(STmp)-OH, Novabiochem®
Nível de qualidade
linha de produto
Novabiochem®
forma
powder
adequação da reação
reaction type: Fmoc solid-phase peptide synthesis
fabricante/nome comercial
Novabiochem®
aplicação(ões)
peptide synthesis
grupo funcional
thiol
temperatura de armazenamento
15-25°C
Categorias relacionadas
Descrição geral
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Fmoc SPPS of Cysteine-Containing Peptides
Literature references:
[1] T. M. Postma, et al. (2012) Org. Lett., 14, 5468.
[2] T. M. Postma & F. Albericio (2013) Org. Lett., 15, 616.
Aplicação
- Synthesis of insulin analogs by regiospecific disulfide bond formation.
- A review on step-wise introduction of disulfide bonds.
- Synthesis of human insulin-like peptide 6.
Nota de análise
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 94.0 % (a/a)
Purity (TLC(011A)): ≥ 98 %
Solubility (1 mmole in 2 ml DMF): clearly soluble
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.05 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
Protocolos
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica