W245115
Ethyl palmitate
natural (US), ≥95%, FG
Sinônimo(s):
Ethyl hexadecanoate, Palmitic acid ethyl ester
About This Item
Produtos recomendados
grau
FG
Fragrance grade
Halal
Kosher
natural (US)
Agency
follows IFRA guidelines
conformidade reg.
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
Ensaio
≥95%
índice de refração
n20/D 1.440 (lit.)
pb
192-193 °C/10 mmHg (lit.)
pf
24-26 °C (lit.)
densidade
0.857 g/mL at 25 °C (lit.)
aplicação(ões)
flavors and fragrances
Documentação
see Safety & Documentation for available documents
alérgeno alimentar
no known allergens
alérgeno de fragrância
no known allergens
Organoléptico
creamy; milk; fruity; waxy
cadeia de caracteres SMILES
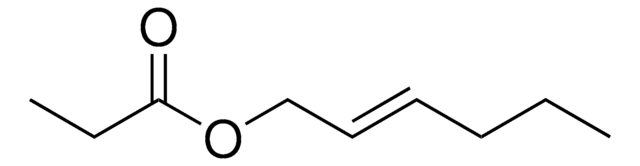
CCCCCCCCCCCCCCCC(=O)OCC
InChI
1S/C18H36O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20-4-2/h3-17H2,1-2H3
chave InChI
XIRNKXNNONJFQO-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica