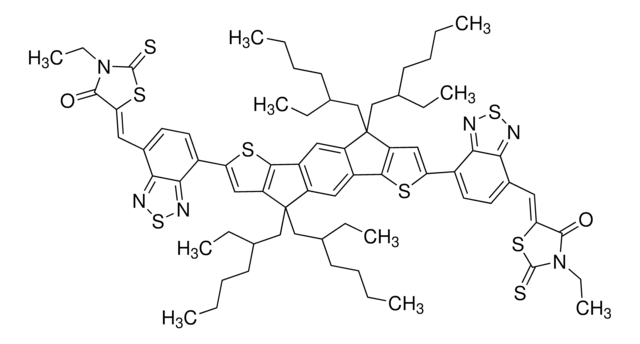
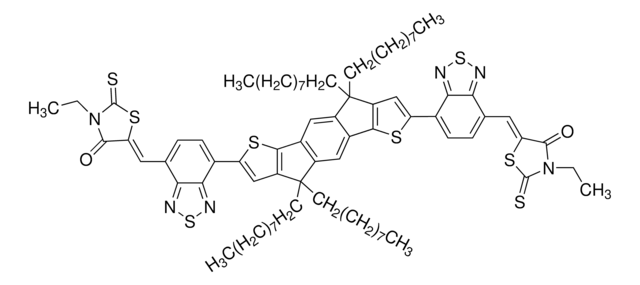
906379
COi8DFIC
≥98%
Sinônimo(s):
2,2′-[[4,4,11,11-tetrakis(4-hexylphenyl)-4,11-dihydrothieno[2′,3′:4,5]thieno[2,3-d]thieno[2′′′′,3′′′′:4′′′,5′′′]thieno[2′′′,3′′′:4′′,5′′]pyrano[2′′,3′′:4′,5′]thieno[2′,3′:4,5]thieno[3,2-b]pyran-2,9-diyl]bis[methylidyne(5,6-difluoro, NFA146, O6T-4F, PCE146
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
descrição
Band gap: 1.62 eV
Ensaio
≥98%
Formulário
solid
solubilidade
soluble (chloroform, CB and ODCB)
Energia orbital
HOMO -5.5 eV
LUMO -3.88 eV
cadeia de caracteres SMILES
Fc1cc2c(cc1F)C(=C(C#N)C#N)\C(=C/c3[s]c4c([s]c5c4C(Oc8c9[s]c%10c(c9[s]c85)OC(c%13c%14[s]c(cc%14[s]c%13%10)\C=C%15/C(=O)c%16c(cc(c(c%16)F)F)C/%15=C(C#N)C#N)(c%12ccc(cc%12)CCCCCC)c%11ccc(cc%11)CCCCCC)(c7ccc(cc7)CCCCCC)c6ccc(cc6)CCCCCC)c3)\C2=O
InChI
1S/C94H76F4N4O4S6/c1-5-9-13-17-21-53-25-33-59(34-26-53)93(60-35-27-54(28-36-60)22-18-14-10-6-2)79-85-75(43-63(107-85)41-69-77(57(49-99)50-100)65-45-71(95)73(97)47-67(65)81(69)103)109-87(79)89-83(105-93)91-92(111-89)84-90(112-91)88-80(86-76(110-88)44-64(10
chave InChI
QPSUTBMJXUUOIC-YTMZJGCBSA-N
Categorias relacionadas
Descrição geral
In a recent study, COi8DFIC or O6T-4F was selected in a Tandem cell by computer assited design and gave a record PCE of 17.3∃% for fabricated organic solar cells.[1]
COi8DFIC or O6T-4F is frequently selected to blend with a narrow-bandgap donor material and another narrow bandgap acceptor material to fabricate ternary organic solar cells[2][3][4]. The PTB7-Th:COi8DFIC:PC71BM ternary cells offered a PCE of 14.08%. By further adopting a post-annealing process, an outstanding PCE of 14.62% can be achieved. Furthermore, the device utilizing COi8DFIC exhibited a good thermal stability with PCEs over 13.5% in a wide temperature range (70–160 °C).[2]
Aplicação
In a recent study, COi8DFIC or O6T-4F was selected in a Tandem cell by computer assited design and gave a record PCE of 17.3% for fabricated organic solar cells.
Tandem Cell Device performance:
ITO/ZnO/PFN-Br/PBDB-T:F-M/M-PEDOT/ZnO/PTB7- Th:O6T-4F:PC71BM/MoO3/Ag
Voc=1.642 V
Jsc=14.35 mA/cm2
FF=73.7%
PCE=17.3%
COi8DFIC or O6T-4F is frequently selected to blend with a narrow-bandgap donor material and another narrow bandgap acceptor material to fabricate ternary organic solar cells. The PTB7-Th:COi8DFIC:PC71BM ternary cells offered a PCE of 14.08%. By further adopting a post-annealing process, an outstanding PCE of 14.62% can be achieved. Furthermore, the device utilizing COi8DFIC exhibited a good thermal stability with PCEs over 13.5% in a wide temperature range (70-160 °C).
Device structure:
ITO/ZnO/PTB7-Th:COi8DFIC:PC71BM/MoO3/Ag
- Before annealing
Jsc=27.74 mA/cm2
FF=0.701
PCE=13.65%
- After annealing at 80°C
Jsc=27.39 mA/cm2
FF=0.734
PCE=14.62%
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica


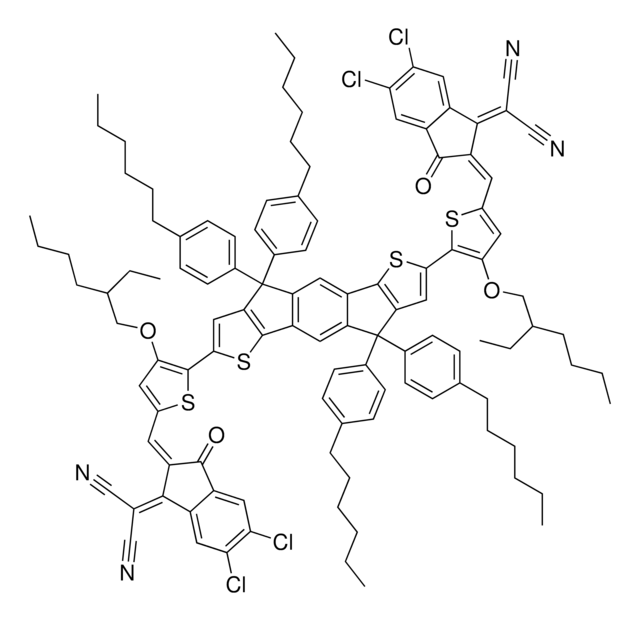




![[6,6]-Phenyl C71 butyric acid methyl ester, mixture of isomers 99%](/deepweb/assets/sigmaaldrich/product/structures/716/624/9fb9f2f0-ae99-429f-8d3a-b12267976a4d/640/9fb9f2f0-ae99-429f-8d3a-b12267976a4d.png)