Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
730998
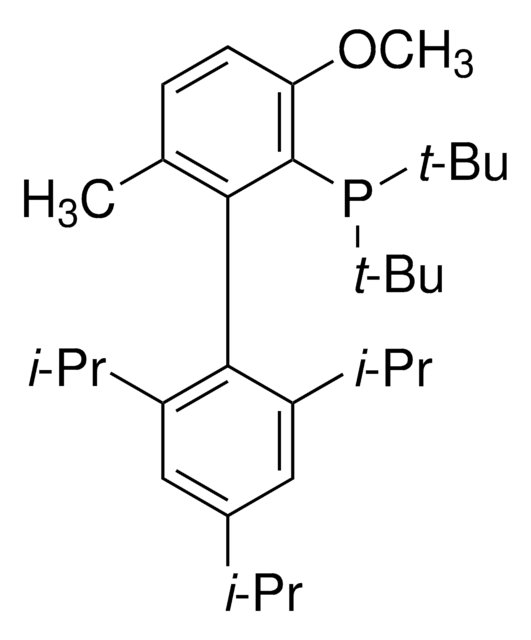
tBuBrettPhos
97%
Sinônimo(s):
t-Bu Brett Phos, t-BuBrett-Phos, tertButylBrettPhos, 2-(Di-tert-butylphosphino)-2′,4′,6′- triisopropyl-3,6-dimethoxy-1,1′-biphenyl, t-BuBrett Phos, t-BuBrettPhos, [3,6-Dimethoxy-2′,4′,6′-tris(1-methylethyl) [1,1′-biphenyl]-2-yl]bis(1,1-dimethylethyl)phosphine, tert-ButylBrettPhos
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
solid
adequação da reação
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Fluorinations
pf
166-170 °C
grupo funcional
phosphine
cadeia de caracteres SMILES
COc1ccc(OC)c(c1P(C(C)(C)C)C(C)(C)C)-c2c(cc(cc2C(C)C)C(C)C)C(C)C
InChI
1S/C31H49O2P/c1-19(2)22-17-23(20(3)4)27(24(18-22)21(5)6)28-25(32-13)15-16-26(33-14)29(28)34(30(7,8)9)31(10,11)12/h15-21H,1-14H3
chave InChI
REWLCYPYZCHYSS-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
tBuBrettPhos is a phosphine ligand widely used in palladium-catalyzed cross-coupling reactions.[1]
Aplicação
- Buchwald-Hartwig amination and C-O coupling
- Suzuki, Negishi, Stille, Hiyama, Sonogashira cross-couplings
- α-Arylation reaction
New Applications:
- Conversion of aryl and vinyl triflates to bromides and chlorides[2]
- Conversion of aryl triflates to aryl fluorides[3]
- O-Arylation of ethyl acetohydroximate[4]
- Conversion of aryl chlorides and sulfonates to nitroaromatics[5]
Características e benefícios
- White crystalline solid
- Air- and moisture-stable
- Thermally stable
- Highly efficient
- Wide functional group tolerance
- Excellent selectivity and conversion
Informações legais
produto relacionado
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Conteúdo relacionado
A plataforma Catalexis aprimora a catálise otimizando digitalmente a seleção do catalisador para identificar os ligantes de fosfina mais eficazes para reações de acoplamento cruzado.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica








![5-(Di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/137/599/8b2f4b58-3384-40aa-9295-0887f7985525/640/8b2f4b58-3384-40aa-9295-0887f7985525.png)