675938
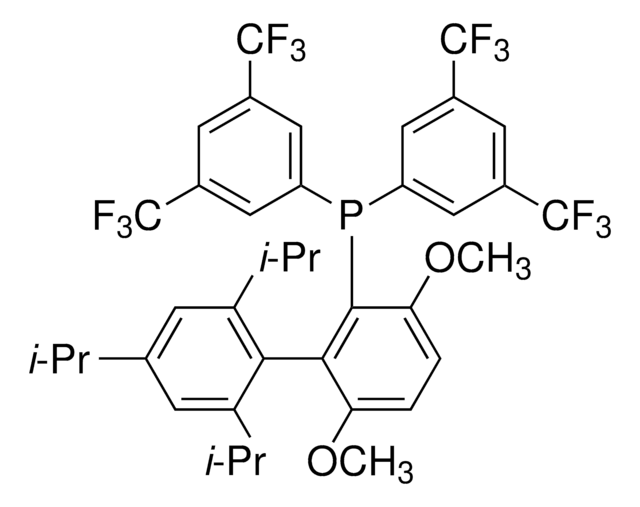
Me4tButylXphos
96%
Sinônimo(s):
2-Di-tert-butylphosphino-3,4,5,6-tetramethyl-2′,4′,6′-triisopropyl-1,1′-biphenyl, Tetramethyl di-tBuXPhos, Tetramethyl tBuXPhos
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
96%
Formulário
solid
adequação da reação
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
pf
168-172 °C
grupo funcional
phosphine
cadeia de caracteres SMILES
CC1=C(C)C(C)=C(C)C(C(C(C(C)C)=CC(C(C)C)=C2)=C2C(C)C)=C1P(C(C)(C)C)C(C)(C)C
InChI
1S/C33H53P/c1-19(2)26-17-27(20(3)4)30(28(18-26)21(5)6)29-24(9)22(7)23(8)25(10)31(29)34(32(11,12)13)33(14,15)16/h17-21H,1-16H3
chave InChI
RCRYEYMHBHPZQD-UHFFFAOYSA-N
Categorias relacionadas
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Buchwald Ligands
Buchwald and coworkers develop versatile phosphine ligands for Pd-catalyzed C–N bond formation; enhancing synthetic reactions for 20 years.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Conteúdo relacionado
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica