799718
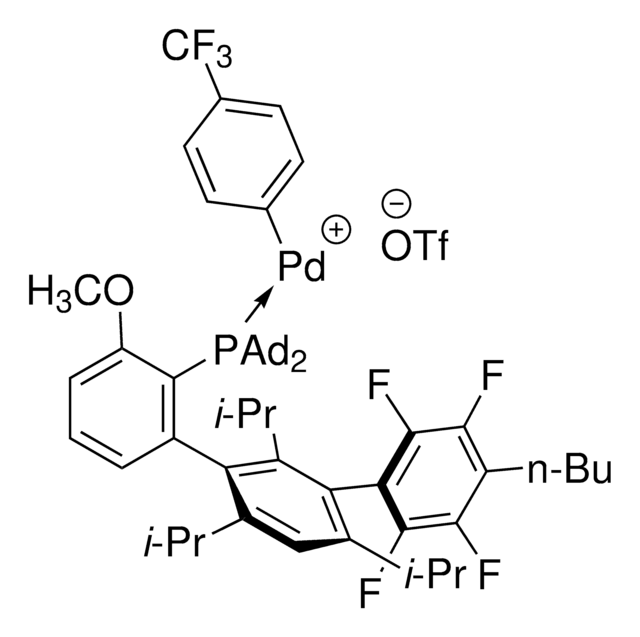
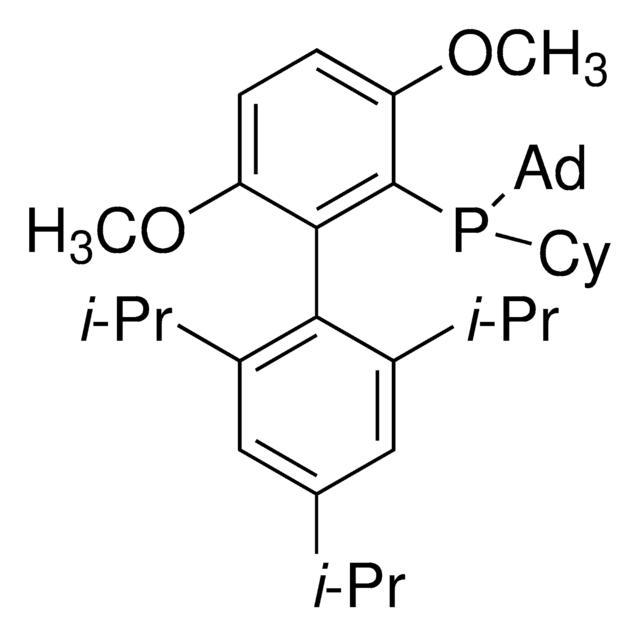
AlPhos
Sinônimo(s):
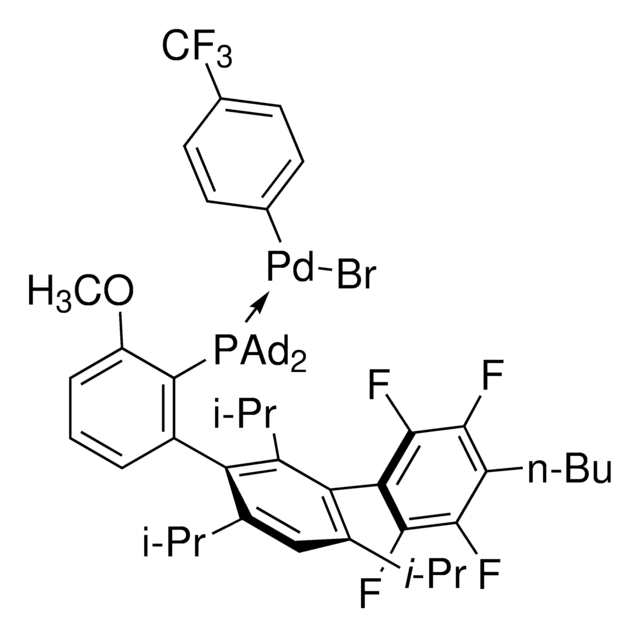
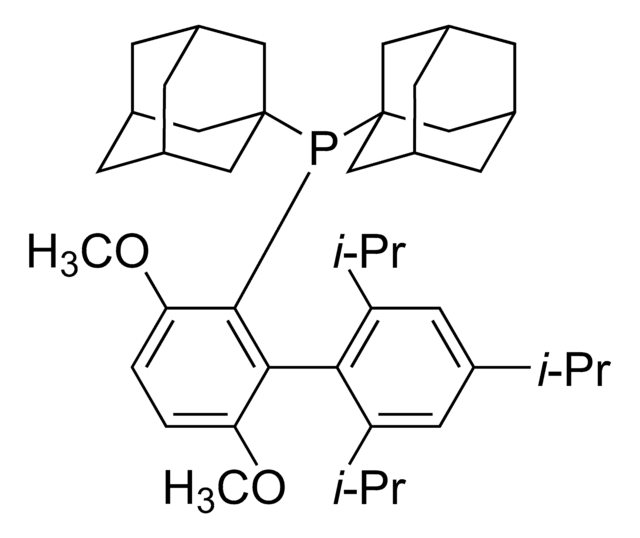
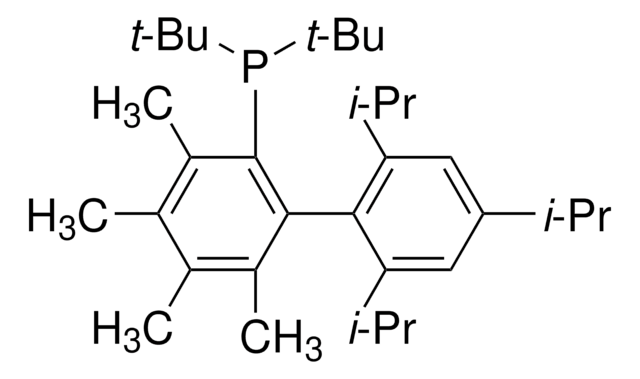
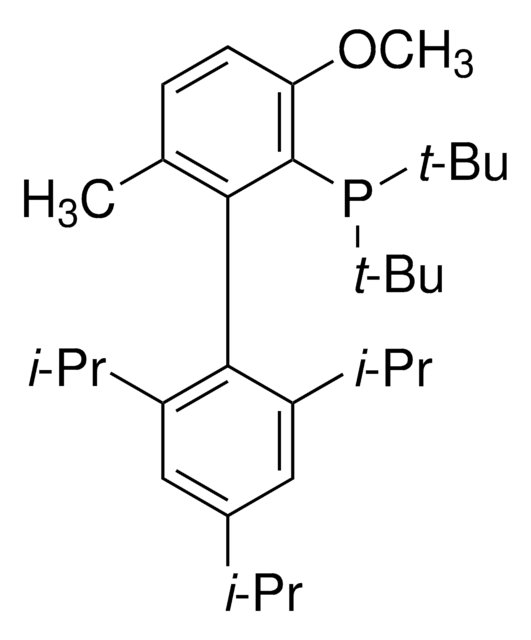
Di-1-adamantyl(4″-butyl-2″,3″,5″,6″-tetrafluoro-2′,4′,6′-triisopropyl-2-methoxy-meta-terphenyl)phosphine
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Ensaio
≥95%
Nível de qualidade
Formulário
powder
adequação da reação
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings
pf
218-223 °C
grupo funcional
phosphine
temperatura de armazenamento
−20°C
InChI
1S/C52H67F4OP/c1-9-10-12-38-46(53)48(55)45(49(56)47(38)54)44-40(29(4)5)21-39(28(2)3)43(42(44)30(6)7)37-13-11-14-41(57-8)50(37)58(51-22-31-15-32(23-51)17-33(16-31)24-51)52-25-34-18-35(26-52)20-36(19-34)27-52/h11,13-14,21,28-36H,9-10,12,15-20,22-27H2,1-8H3/t31-,32+,33?,34-,35+,36?,51+,52?,58?
chave InChI
ALWIRDZSIXWCBO-VABCSHEKSA-N
Categorias relacionadas
Aplicação
- In the Pd-catalyzed Buchwald-Hartwig cross-coupling reactions.[1]
- To synthesize highly regioselective aryl fluorides by Pd-catalyzed fluorination of a variety of activated aryl and heteroaryl triflates and bromides.[2][3]
- To prepare aryl thioethers by C–S cross-coupling of thiols with aromatic electrophile in the presence of palladium catalyst.[4]
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Fluorine containing aromatics (ArF) are desirable compounds with applications in medicinal chemistry and the agricultural industry.
Conteúdo relacionado
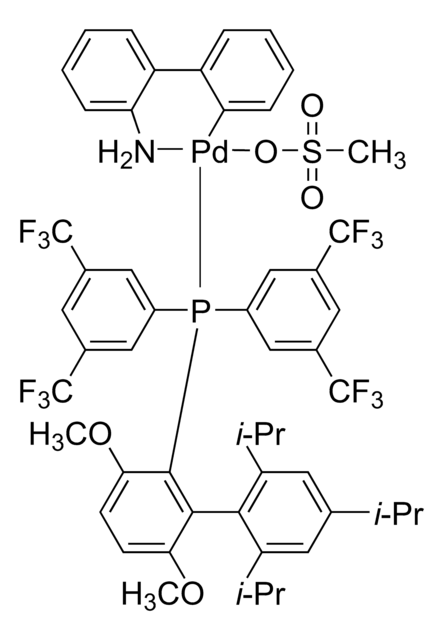
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica