421987
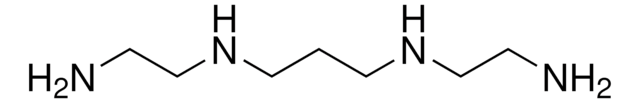
Bis(hexamethylene)triamine
technical grade, 40%
Sinônimo(s):
6,6′-Iminodihexylamine, Bis(6-aminohexyl)amine
About This Item
Produtos recomendados
grau
technical grade
pressão de vapor
<0.01 mmHg ( 25 °C)
concentração
40%
índice de refração
n20/D 1.49 (lit.)
p.e.
163-165 °C/4 mmHg (lit.)
pf
33-36 °C (lit.)
densidade
0.85 g/mL at 20 °C (lit.)
0.931 g/mL at 25 °C
cadeia de caracteres SMILES
NCCCCCCNCCCCCCN
InChI
1S/C12H29N3/c13-9-5-1-3-7-11-15-12-8-4-2-6-10-14/h15H,1-14H2
chave InChI
MRNZSTMRDWRNNR-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica


![Trimethylolpropane tris[poly(propylene glycol), amine terminated] ether average Mn 440](/deepweb/assets/sigmaaldrich/product/structures/186/658/1b1d510a-705a-4bfd-b90a-9dec80d64467/640/1b1d510a-705a-4bfd-b90a-9dec80d64467.png)