18886
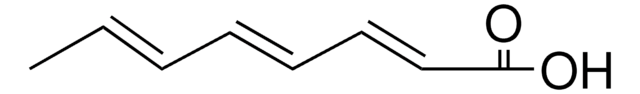
2,4-Pentadienoic acid
≥97.0% (T)
Sinônimo(s):
1,3-Butadiene-1-carboxylic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH2=CHCH=CHCOOH
Número CAS:
Peso molecular:
98.10
Beilstein:
1739248
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
≥97.0% (T)
Formulário
solid
contém
hydroquinone as stabilizer
pf
69-72 °C
solubilidade
1 M NaOH: soluble 0.5 g/10 mL, clear, brown
grupo funcional
carboxylic acid
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
OC(=O)\C=C\C=C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h2-4H,1H2,(H,6,7)/b4-3+
chave InChI
SDVVLIIVFBKBMG-ONEGZZNKSA-N
Aplicação
2,4-Pentadienoic acid was used in preparation of chiral propargylester. It was also used in the preparation of trans 1-N-acylamino-1,3-dienes via modified Curtius procedure.
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Xiaohu Yi et al.
ChemSusChem, 10(7), 1494-1500 (2017-01-18)
A series of choline (Ch)-exchanged heteropoly acids (HOCH
Daichi Oguro et al.
Bioscience, biotechnology, and biochemistry, 75(8), 1502-1505 (2011-08-09)
The synthesis and sensory evaluation of enantiomeric sets of sedanenolide (1) and 3-butylphthalide (3) are described. The asymmetric synthesis was achieved via the intramolecular Diels-Alder reaction of chiral propargylester (5) which was prepared from optically active propargyl alcohol (4) and
trans-1-N-Acylamino-1, 3-dienes: preparation from dienoic acids.
Overman L, et al.
The Journal of Organic Chemistry, 43(11), 2164-2167 (1978)
Brian D Hudson et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(12), 4951-4965 (2012-08-25)
When it is difficult to develop selective ligands within a family of related G-protein-coupled receptors (GPCRs), chemically engineered receptors activated solely by synthetic ligands (RASSLs) are useful alternatives for probing receptor function. In the present work, we explored whether a
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica